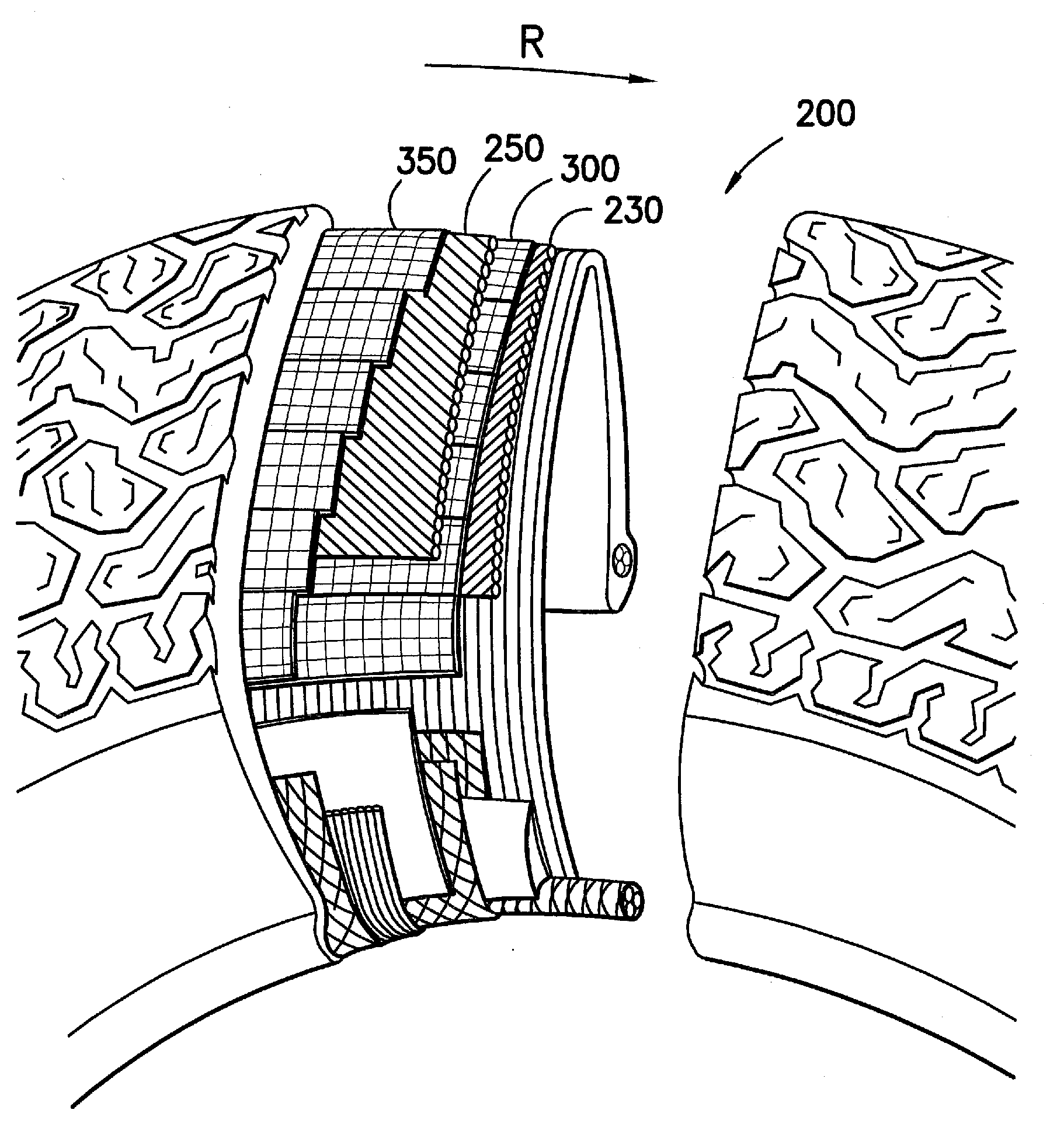
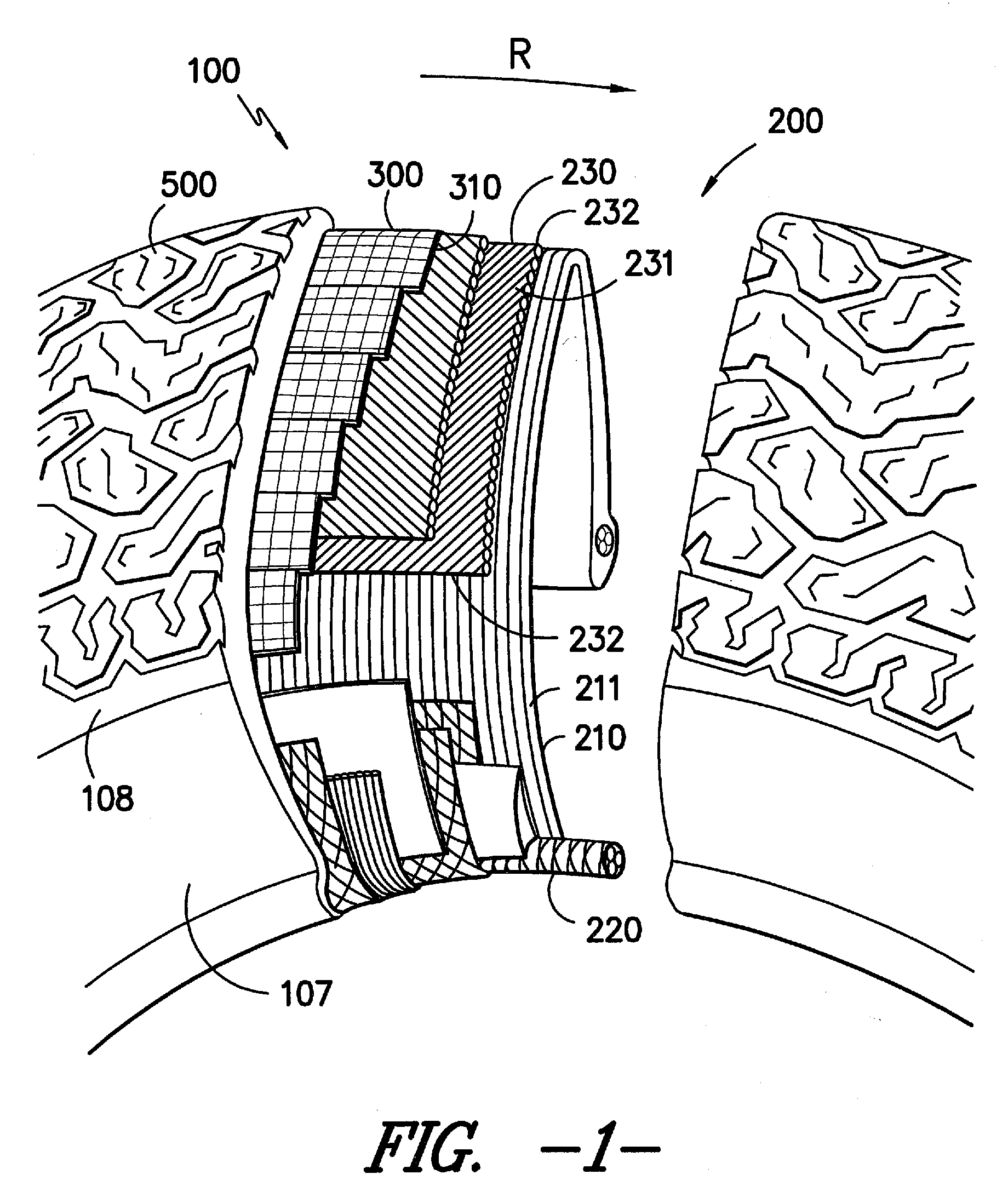
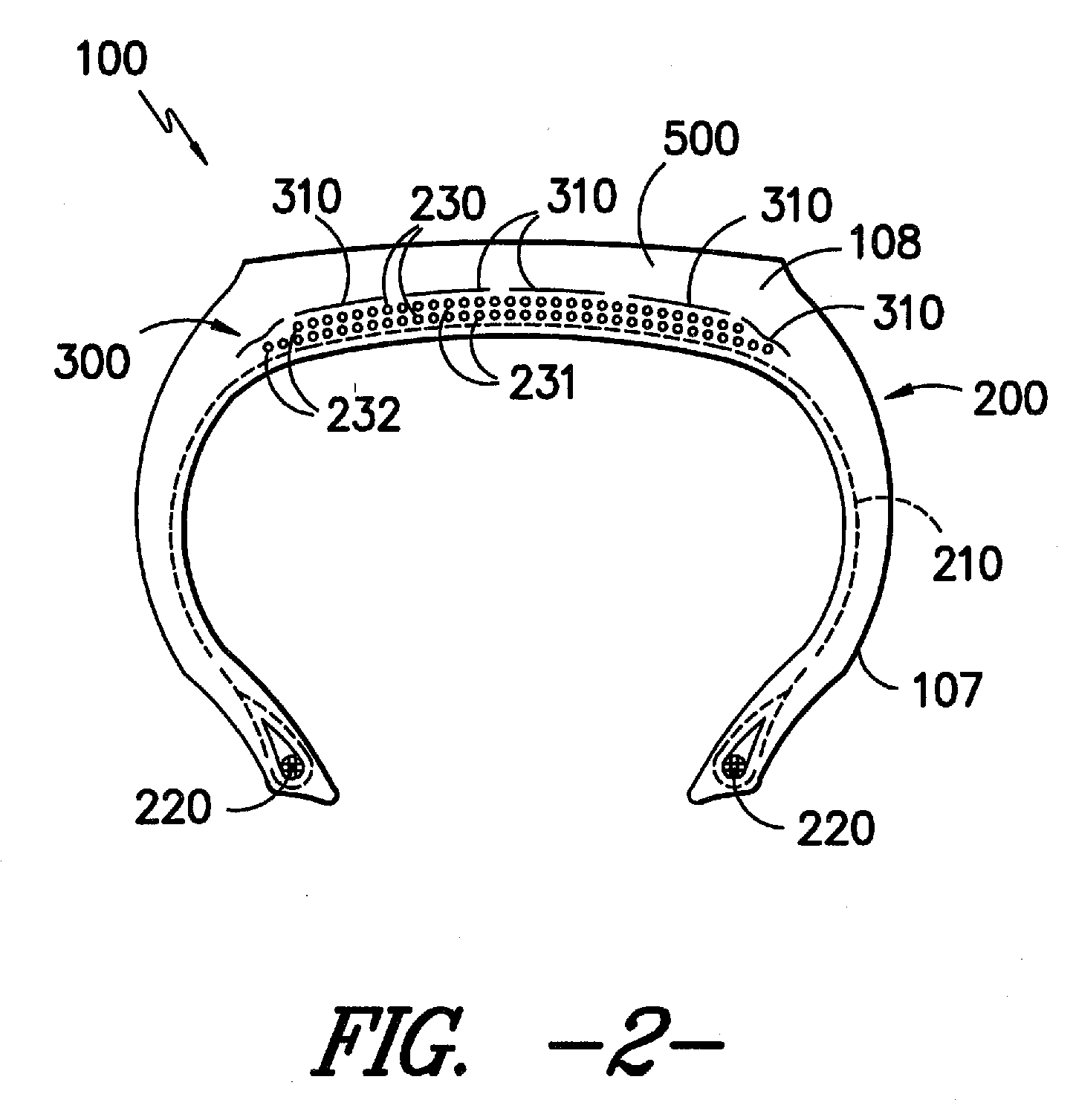
Adhesion Composition and Textile Materials and Articles Treated Therewith
a technology of textile materials and compositions, applied in the direction of aldehyde/ketone condensation polymer adhesives, woven fabrics, tyre parts, etc., can solve the problems of inhalation of potential health hazards, loss of formaldehyde in a convection oven, and multiple health hazards of formaldehyde in traditional rfl compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0109]In general, each formulation was made under ambient conditions according to the procedures described herein. However, furfuraldehyde-based formulations may be made and aged at slightly elevated temperature, such as from about 40° C. to about 60° C.
[0110]In a typical procedure, a small amount of base (typically NaOH and / or ammonium hydroxide) was first dissolved in a given amount of water. The phenolic condensate component (such as resorcinol-formaldehyde condensate or furfuraldehyde-resorcinol condensate) was added to the base solution under stirring until a homogeneous solution was made. The aldehyde component was slowly added to the phenolic condensate under stirring. The homogeneous solution was slowly poured into the latex component, such as vinylpyridine rubber latex, under stirring to form a mixture. The mixture was kept in a closed container at ambient temperature for about 12 to 72 hours. Other optional additives, such as antioxidants, carbon black, silica, thickeners,...
example 2
[0113]
Formulation 2IngredientAmount (parts by weight)Chemisat ® LCH 730270(hydrogenated nitrile rubber latex,Zeon Chemicals)Penacolite ® 21704.5(from Indspec Chemical Corporation)2-Furfuraldehyde0.8(from Aldrich)Water20Ammonium hydroxide, 37%1.4(from Aldrich)Paragum 1847(from Parachem)
[0114]Formulation 2 was applied to Fabric A as described herein. The treated fabric was then combined with rubber sheets for form a textile substrate-rubber composite as described herein. The peel force was measured to be about 30 lb / in for Example 2.
example 3
2-Furfuraldehyde-resorcinol Condensate
[0118]4 g of resorcinol (Aldrich) was dissolved in 11 g of water that contained 0.5 g of 50% sodium hydroxide solution. 3.9 g of 2-furfuraldehyde was then added slowly to the resorcinol-water-base solution. This mixture was heated to about 80° C. under stirring and maintained at 80° C. for about 2 hours. The mixture was then cooled to room temperature to yield a viscous solution of the condensate. This condensate was then incorporated into the following formulation:
Formulation 3IngredientAmount (grams)Condensate obtained from above13Deionized Water51.9Caustic solution (50%)0.62-furfuraldehyde2
[0119]The above mixture was added to a solution prepared from 109.6 grams of Gentac® 118 (vinyl pyridine rubber latex from Omnova Solutions Inc.) and 24 grams of water.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com