Production of extracellular matrix, conditioned media and uses thereof
a technology of conditioned media and extracellular matrix, which is applied in the direction of manufacturing tools, drug compositions, skeletal/connective tissue cells, etc., can solve the problems of affecting post-attachment events, cell death or inability of cells to differentiate, and existing therapy has significant limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
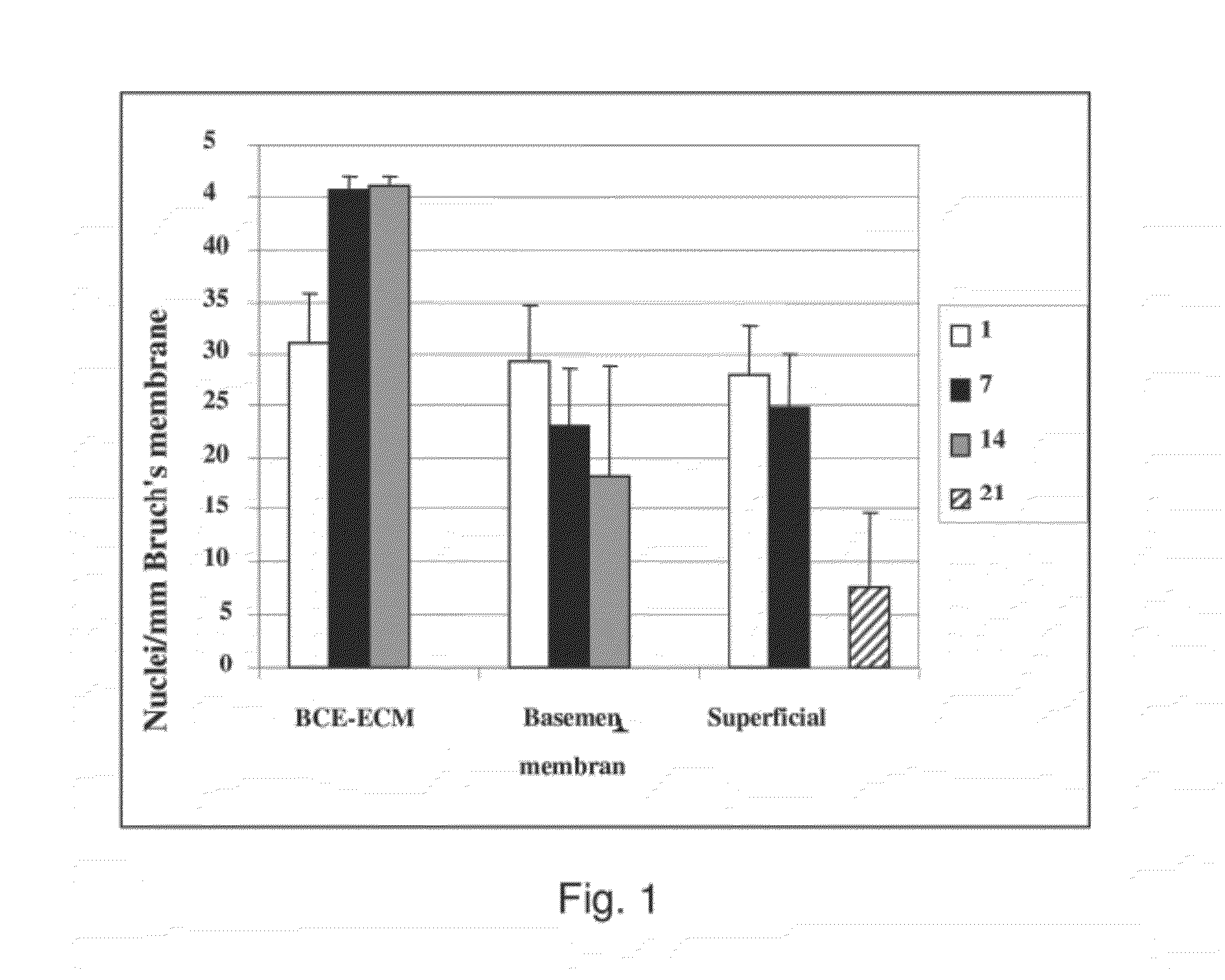
Long-Term Survival of Fetal RPE on Aged Submacular Human Bruch's Membrane is Impaired
[0129]Fetal RPE (3164 cells / mm2) were seeded on aged human submacular Bruch's membrane debrided to expose the superficial surface of the inner collagenous layer. To create surfaces exposing the RPE basement membrane, RPE were gently wiped off the RPE / choroid / sclera explant using a wet surgical sponge. To create surfaces exposing the surface of the inner collagenous layer beneath the RPE basement membrane (i.e., superficial ICL), following RPE removal as indicated previously, a moistened surgical sponge was use to abrade the RPE basement membrane. In general, the area of RPE basement membrane debridement was created by approximately 5 wipes of the moistened sponge in each of 4 directions (rotating the explant 90 degrees after each series of 5 wipes). (V. K. Gullapalli, et al., Exp Eye Res 2005; 80(2):235-248). Cells were seeded onto the sclera / choroid explant and cultured for 21 days and evaluated fo...
example 2
Fetal RPE Resurfacing on Aged Bruch's Membrane Resurfaced with Bovine Corneal Endothelial Matrix (BCE-ECM)
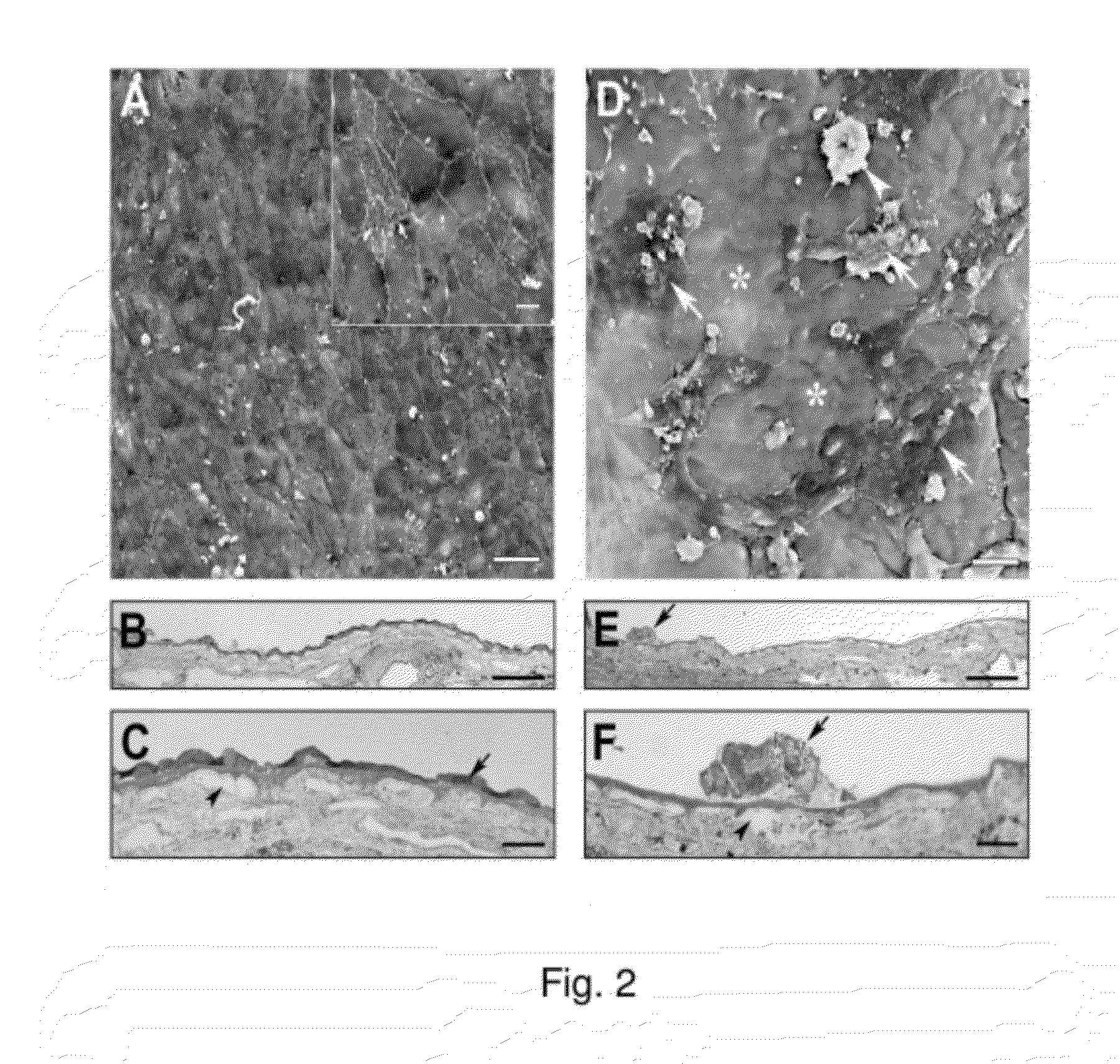
[0130]BCE (3164 cells / mm2) were cultured on the inner collagenous layer of aged human submacular Bruch's membrane (65 yr. old donor) for 14 days to allow ECM deposition. Cells were culture in the same way as cells cultured for ECM deposition on culture dishes (see paragraph 0056). Following BCE removal with NH4OH to expose the newly deposited ECM and extensive washing with PBS, explants were seeded with fetal RPE (3164 cells / mm2) and cultured for 21 days. The results of these experiments are illustrated in FIG. 2.
[0131]FIG. 2A is a scanning electron micrograph (SEM), showing that fetal RPE fully resurfaced the treated explant with large, flat polymorphic cells. Cells showed varying amounts of short apical processes on their surfaces (insert). Mag. bar 50 μm; insert mag bar 10 μm.
[0132]FIGS. 2B and 2C are light micrographs (LMs). As shown in FIG. 2B, cells fully resurfaced the tr...
example 3
Resurfacing Bruch's Membrane with a Biologically Deposited Extracellular Matrix (ECM) Improves Cell Survival
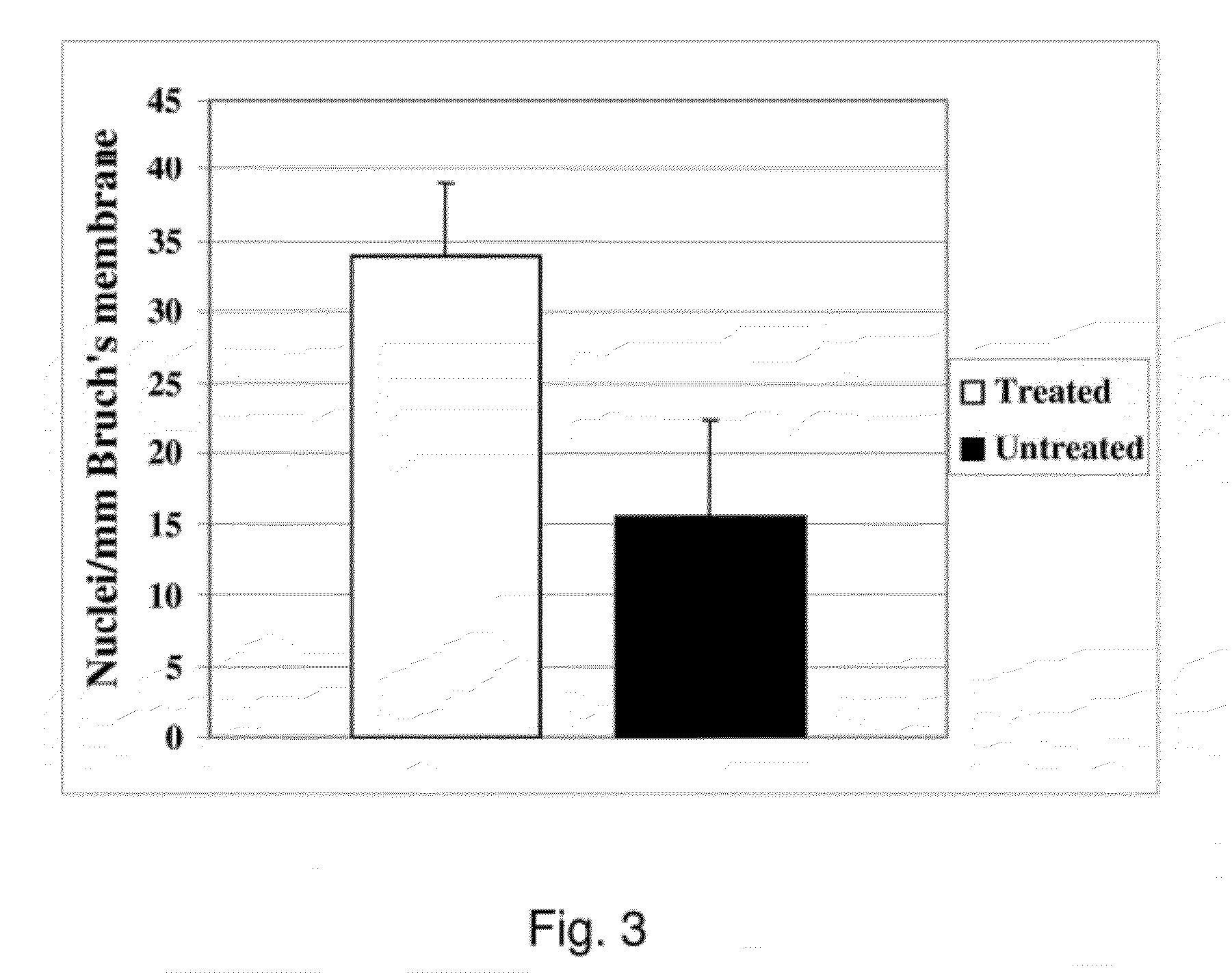
[0136]Bovine corneal endothelial cells (BCE, passage-2) were seeded onto human submacular superficial ICL of Caucasian donors over 55 years old at a density of 3164 cells / mm2 and cultured for 14 days to allow ECM deposition or treated for 14 days with serum-free media only. Following BCE removal with NH4OH and extensive rinsing, fetal RPE (passage-2-5) were seeded at the same density onto the treated Bruch's membrane surface and cultured for 21 days. The fellow eye was treated similarly except no BCE were seeded.
[0137]RPE seeding density was 3164 cells / mm2 for 21-day incubations to determine long-term survival and morphology. FIG. 3 shows the cumulative data from 9 explant pairs. Counts are mean fetal RPE nuclei / mm Bruch's membrane (±SEM).
[0138]A statistically significant 230% (p=0.006) increase in cell density is seen at day-21 on treated explants compared to explants treated...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com