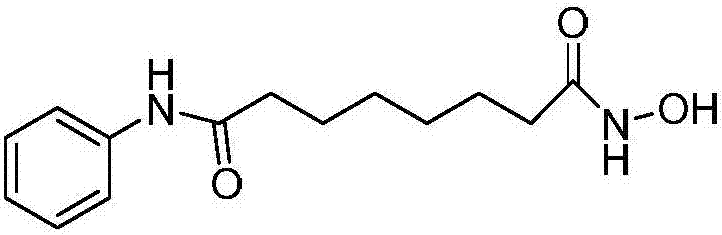
A kind of preparation method of anticancer drug vorinostat
A technology of vorinostat and anticancer drugs, which is applied in the field of preparation of anticancer drug vorinostat, can solve the problems of low product yield, long reaction time, poor selectivity, etc., and achieve good selectivity and high yield , the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of suberic acid-substrate self-assembled film
[0027] Hydrophilic treatment of quartz sheet: place the cut substrate (quartz sheet, 10cm×10cm) in a strong acid mixed solution (H 2 SO 4 / HNO 3 , volume ratio 1:1), boiled at 90°C for 1 hour, then stood at room temperature, cleaned with deionized water and ultrasonicated for 15 minutes to obtain a hydrophilic substrate, which was stored in deionized water until use.
[0028] The specific process of self-assembly includes: adding the hydrophilic substrate to the solution of suberic acid in anhydrous toluene, keeping it warm at 50°C for 10 hours, then taking out the substrate and washing it with water to obtain the suberic acid-substrate self-assembled film . The solution concentration of suberic acid in anhydrous toluene is 1×10 -3 mol / L.
Embodiment 2
[0030] Preparation of suberic acid-substrate self-assembled film
[0031] Hydrophilic treatment of quartz wafers: place the cut substrate (silicon wafer, 10cm×10cm) in a strong acid mixed solution (H 2 SO 4 / HNO 3 , volume ratio 1:1), boiled at 90°C for 1 hour, then stood at room temperature, cleaned with deionized water and ultrasonicated for 15 minutes to obtain a hydrophilic substrate, which was stored in deionized water until use.
[0032] The specific process of self-assembly includes: adding the hydrophilic substrate to the solution of suberic acid in anhydrous toluene, keeping it warm at 60°C for 8 hours, then taking out the substrate and washing it with water to obtain the suberic acid-substrate self-assembled film . The solution concentration of suberic acid in anhydrous toluene is 1×10 -4 mol / L.
Embodiment 3
[0034] Preparation of suberic acid-substrate self-assembled film
[0035] Hydrophilic treatment of quartz sheet: place the cut substrate (glass sheet, 10cm×10cm) in a strong acid mixed solution (H 2 SO 4 / HNO 3 , volume ratio 1:1), boiled at 90°C for 1 hour, then stood at room temperature, cleaned with deionized water and ultrasonicated for 15 minutes to obtain a hydrophilic substrate, which was stored in deionized water until use.
[0036] The specific process of self-assembly includes: adding the hydrophilic substrate to the solution of suberic acid in anhydrous toluene, keeping it warm at 45°C for 10 hours, then taking out the substrate and washing it with water to obtain the suberic acid-substrate self-assembled film . The solution concentration of suberic acid in anhydrous toluene is 1×10 -3 mol / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com