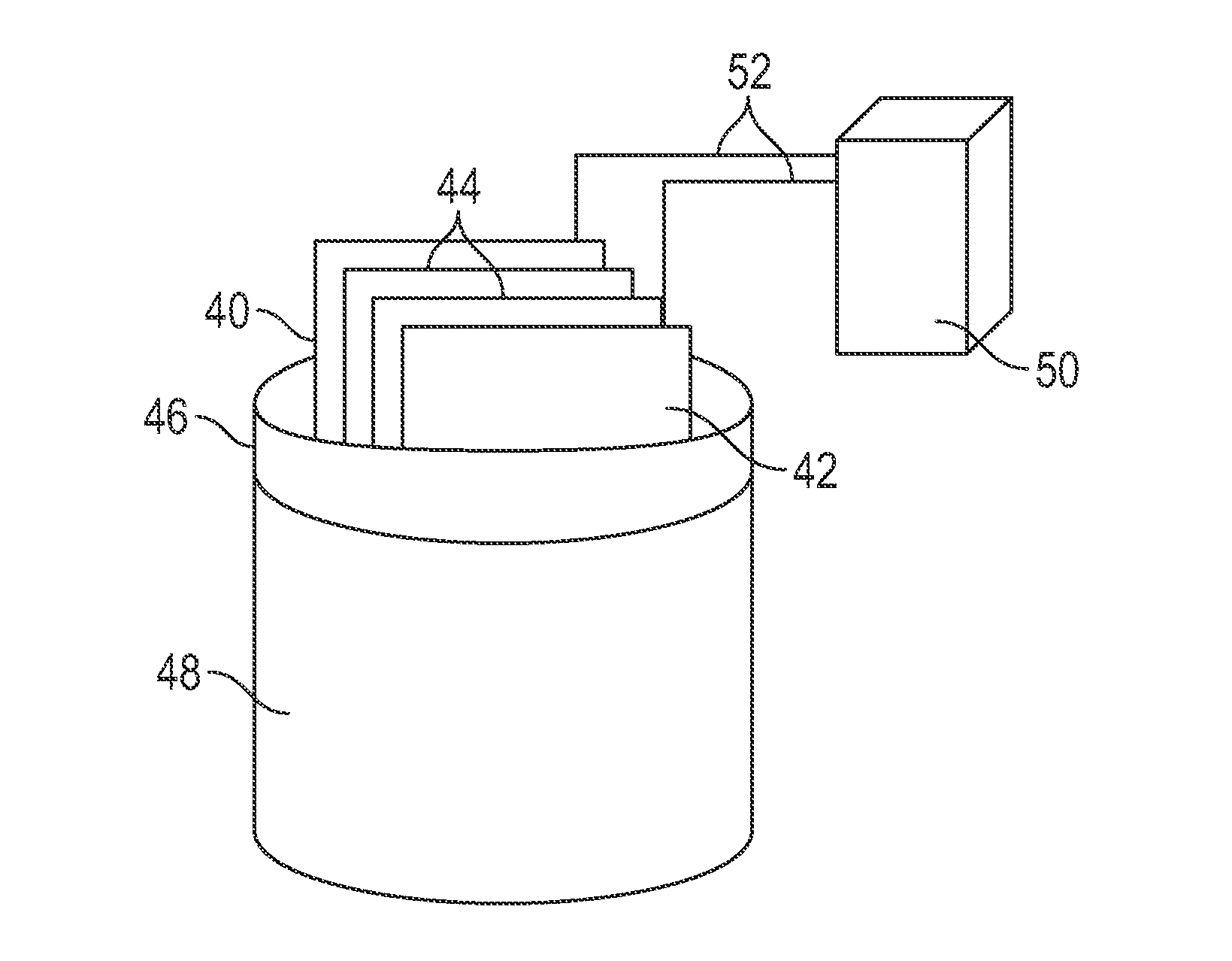
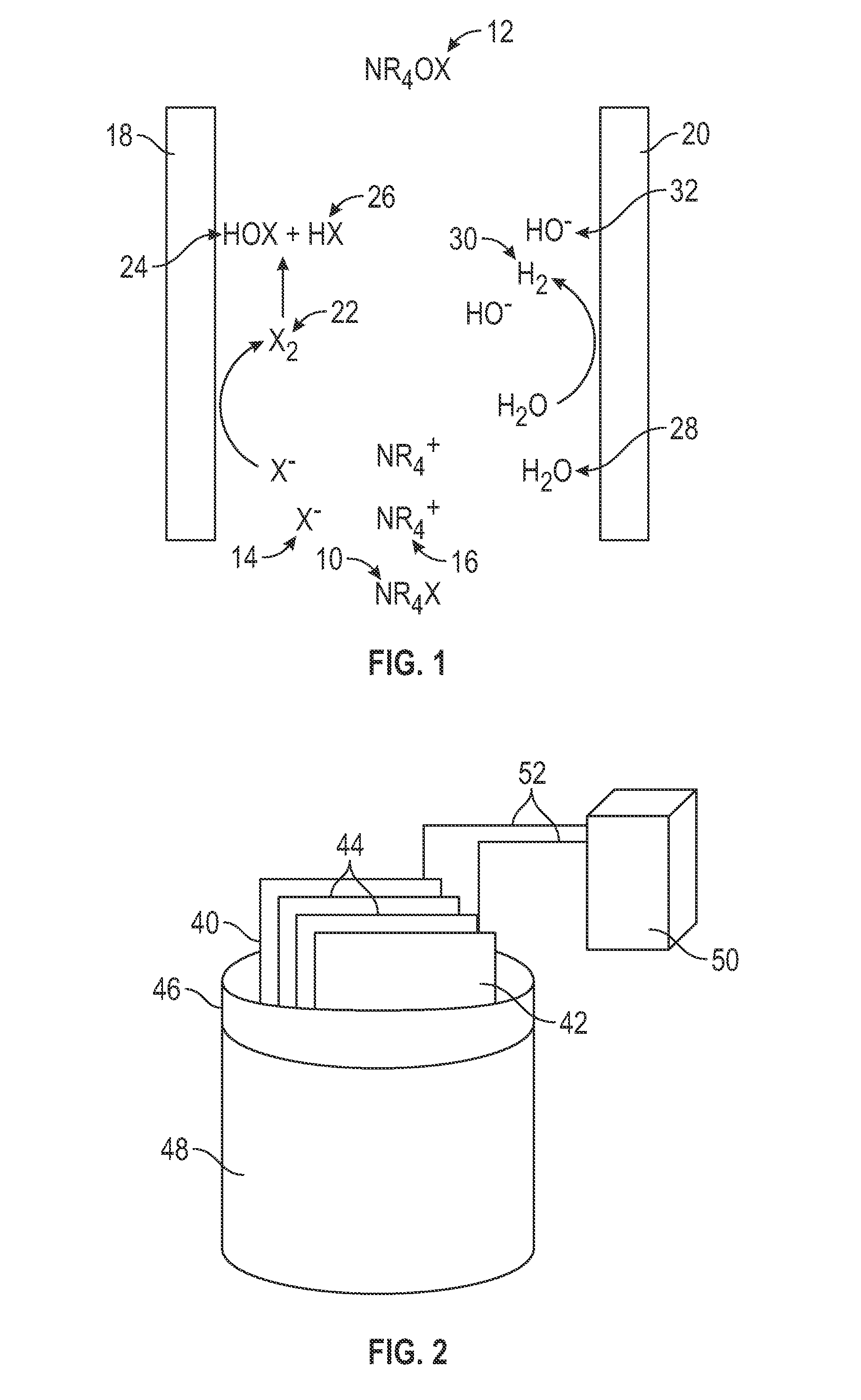
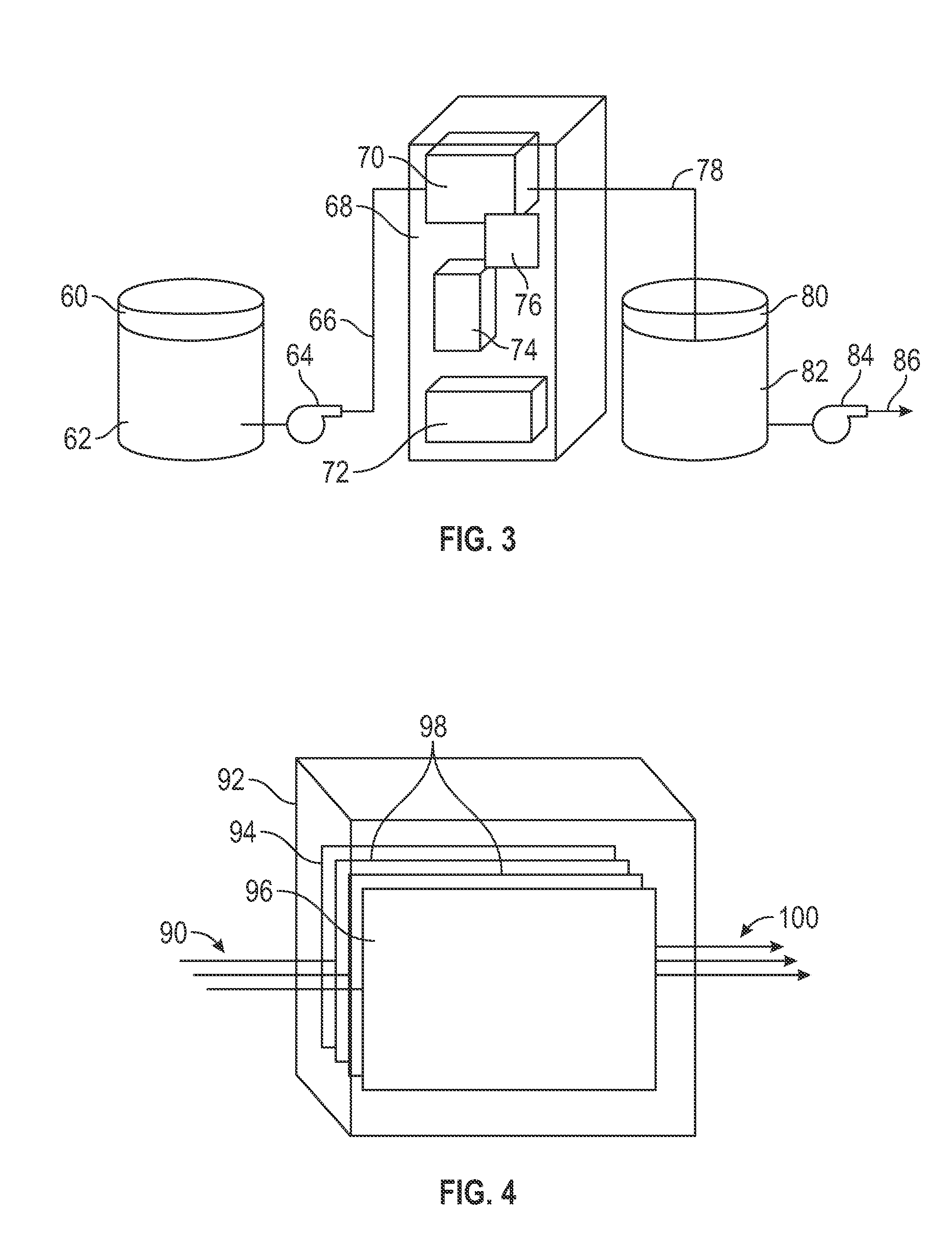
Electrochemical Generation of Quaternary Ammonium Compounds
a technology of quaternary ammonium and halide salts, which is applied in the production of electrolytic organic products, manufacturing tools, electrolysis components, etc., can solve the problems of reducing the overall combination effect, adversely affecting the stability of compounds and solutions, and the decomposition of combined solutions typically degrades quickly, so as to achieve the effect of increasing the disinfection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0048]An aqueous solution of benzalkonium chloride was prepared by dissolving 10 grams of benzalkonium chloride in 1000 mL of deionized water to give a 1% (10,000 mg / L) solution. 200 mL of this solution was transferred to a 250 mL beaker, and a three electrode cell was immersed in the solution. The cell was then energized to 12 volts for 20 minutes. During this time, extensive bubbling was observed on the cathodic electrodes, producing a foam above the surface of the solution (similar to that shown in FIGS. 10a and 10b). After 20 minutes, a sample of the electrolyzed solution was withdrawn and the free available chlorine (FAC) content of the solution was determined to be 332 mg / L using the diethyl-p-phenylene diamine (DPD) method.
example 2
[0049]An aqueous solution of cetyltrimethylammonium chloride was prepared by adding 20 mL of a 25% by weight cetyltrimethylammonium solution to 1000 mL of deionized water to give a 0.5% (5,000 mg / L) solution. 200 mL of this solution was transferred to a 250 mL beaker, and a three electrode cell was immersed in the solution. The cell was then energized to 12 volts for 60 minutes. During this time, extensive bubbling was observed on the cathodic electrodes, producing a foam above the surface of the solution (similar to that shown in FIGS. 10a and 10b). After 60 minutes, a sample of the electrolyzed solution was withdrawn and the free available chlorine content of the solution was determined to be 360 mg / L using the DPD method.
example 3
[0050]A solution containing 100 mg / L benzalkonium chloride and 5,000 mg / L sodium chloride was prepared by dissolving 0.1 g benzalkonium chloride and 5 g sodium chloride in 1000 mL deionized water. 200 mL of this solution was transferred to a 250 mL beaker, and a three electrode cell was immersed in the solution. The cell was then energized to 12 volts for 20 minutes. During this time, extensive bubbling was observed on the cathodic electrodes, producing a foam above the surface of the solution (similar to that shown FIGS. 10a and 10b). After 20 minutes, a sample of the electrolyzed solution was withdrawn and the free available chlorine content of the solution was determined to be 3,000 mg / L using the DPD method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| chemical structure | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com