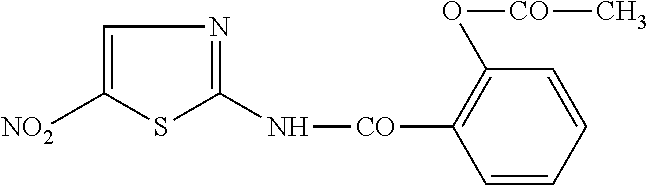
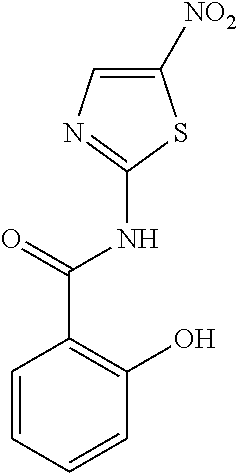
Use of thiazolide compounds for the prevention and treatment of viral diseases, cancer and diseases caused by intracellular infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cell Preparation
[0056]Mononuclear blood cells have an important role in the immune response system, as they produce different cytokines in response to pathogen infections. Accordingly, the immunomodulatory effects of tizoxanide (TIZ) in peripheral mononuclear blood cells (PMBCs) obtained from ten (10) healthy donors and isolated by centrifugation on Ficoll-Paque. The PMBCs were cultured in RPMI-1640 media supplemented with 10% human serum in the presence or absence of three different doses of TIZ (0.5, 1.0 and 10 mg / ml) in both unstimulated and flu-stimulated conditions.
example 2
Immunological Analyses
[0057]The unstimulated and stimulated PMBCs were analyzed for T helper and CTL activity as well as for TLR7 and TLR8 expression and type I IFN responses in the absence or presence of different doses of tizoxanide. The immunological analyses were as follows:
[0058]T helper functions were detected by determining the amount of IFNγ- and IL-2-secreting CD4+ T cells. CTL activity was detected by determining the amount of perforin-, granzyme- and Fas-expressing CD8+ T cells.
[0059]TLR expression was detected by measuring TLR8-, TLR7- and TLR3-expressing CD14+ monocytes. Tizoxanide modulation of the TLR pathway was detected by PCR array analysis of Human Type I Interferon (IFN).
[0060]Specifically, TIZ immunomodulating effects were determined in unstimulated and flu-stimulated PMBCs by analysis of the following:
[0061]Human Type I Interferon (IFN) and TLR Pathway (PCR Array):
Interferons: Ligands for Interferon-alpha and Interferon-beta Receptors: IFNA1, IFNA4, IFNB1, IFNK...
example 3
The Immunomodulatory Effects of Tizoxanide
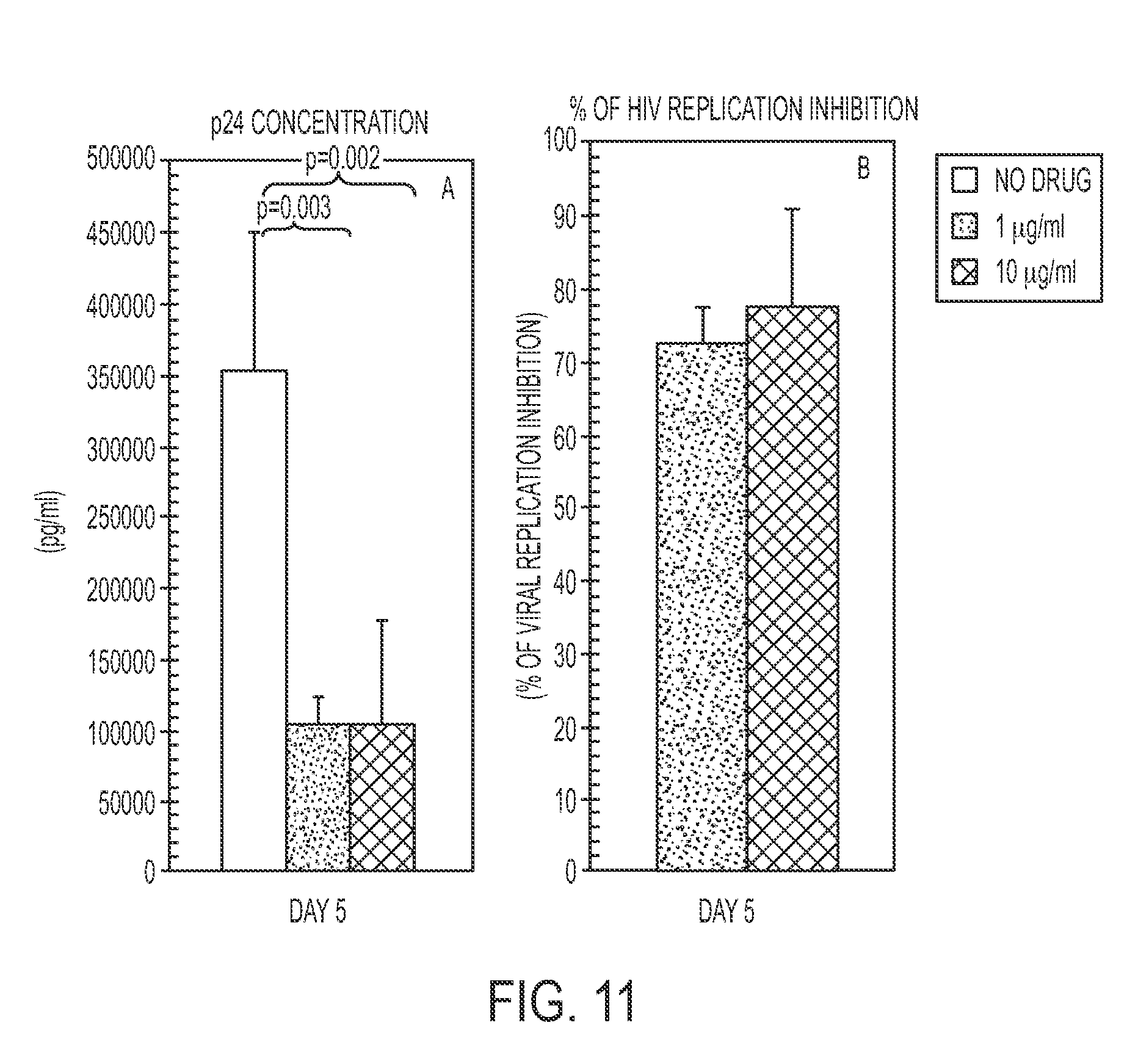
[0070]TIZ showed potent immunomodulatory effects inducing an increase in: 1) IFNγ- and IL2-secreting CD4+ T cells (FIGS. 4A and 4B); 2) CTL degranulation (FIG. 5B); 3) Fas-expressing CD8+ T cells (FIG. 5C); 4) TLR3-, TLR8- and TLR7- expression on monocytes (FIGS. 1A-C); 5) IFNα- and IFNβ- mRNA expression (FIG. 3A), 6) mRNA specific for type I IFN inducible genes (MXA, PRKCZ, ADAR, CXCL10, IRF1, PRKRA) (FIG. 3B); and 7) mRNA specific for gene involved in MHC class I presentation (HLA-A, HLA-B, TAP1) (FIG. 3C).
[0071]These results clearly demonstrate that TIZ has remarkable immumodulatory activity and stimulates a strong immune response, which is mediated by both the innate and the acquired immune systems.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Immunogenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com