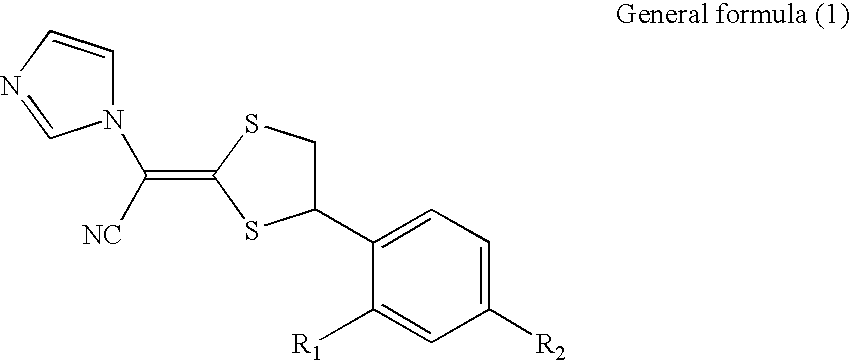
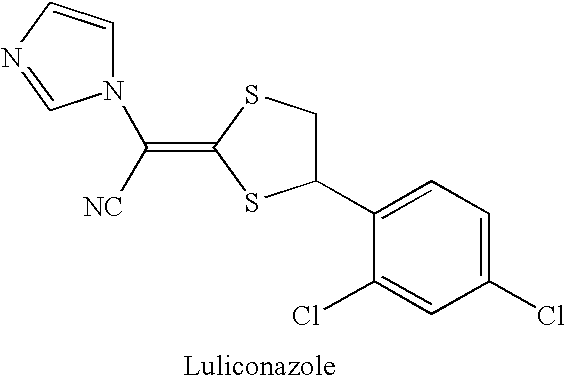
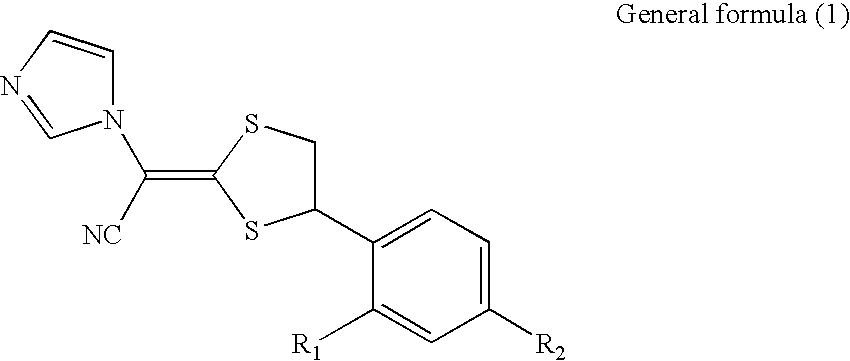
Antimycotic pharmaceutical composition
a technology of mycotic and pharmaceutical composition, applied in the direction of antibacterial agents, pedicures, biocide, etc., can solve the problems of limited amount of solvents, and limited use of solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0040] Hereinafter, the present invention is described in more detail by way of examples.
examples 1 to 7
[0041] Pharmaceutical composition for external use of the present invention was produced in accordance with the formulations shown in Table 1 below. That is, luliconazole was wetted with a part (10 to 30% by mass) of dehydrated ethanol, lactic acid as a component for suppressing crystallization was dissolved in the remainder of dehydrated ethanol, and those were mixed and dissolved with heating (at 50° C. to 90° C.). To the mixture under heating (at 80 to 90° C.) successively added were benzyl alcohol and propylene carbonate. After confirmation of solubilization, acetone was added, the remainder of components was added, and the whole was mixed with stirring. After confirmation of solubilization, the mixture was cooled with stirring to afford Pharmaceutical compositions 1 to 5 for external use of the present invention. After preservation at 20° C. for 12 hours of those compositions, no crystal deposition was confirmed in observation with the naked eye and under a microscope, and clea...
example 8
[0048] Components of Table 3 below were treated, and a pharmaceutical composition for external use of Example 8 was obtained in the same method as in Example 1. In the pharmaceutical composition, such crystal deposition that is less than Comparative Example 1 but was recognized only under a microscope was confirmed under a constant temperature of 20° C. immediately after manufacture. It should be noted that, when the pharmaceutical composition was preserved at 5° C., such a small amount of crystal deposition that was recognized only under a microscope was confirmed on Day 1. As long as the solubility is concerned, it was revealed that the diester of a dibasic acid exhibited a similar effect to that of diester carbonate.
TABLE 3Formulation (% by mass)ComponentsExample 8Luliconazole10Acetone10Propylene carbonate—Benzyl alcohol 2DID12Lactic acid 4Propylene glycol—Dehydrated ethanol62Presence or absence of crystal depositionΔ (small amount ofimmediately after manufacturecrystal deposit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| constant temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com