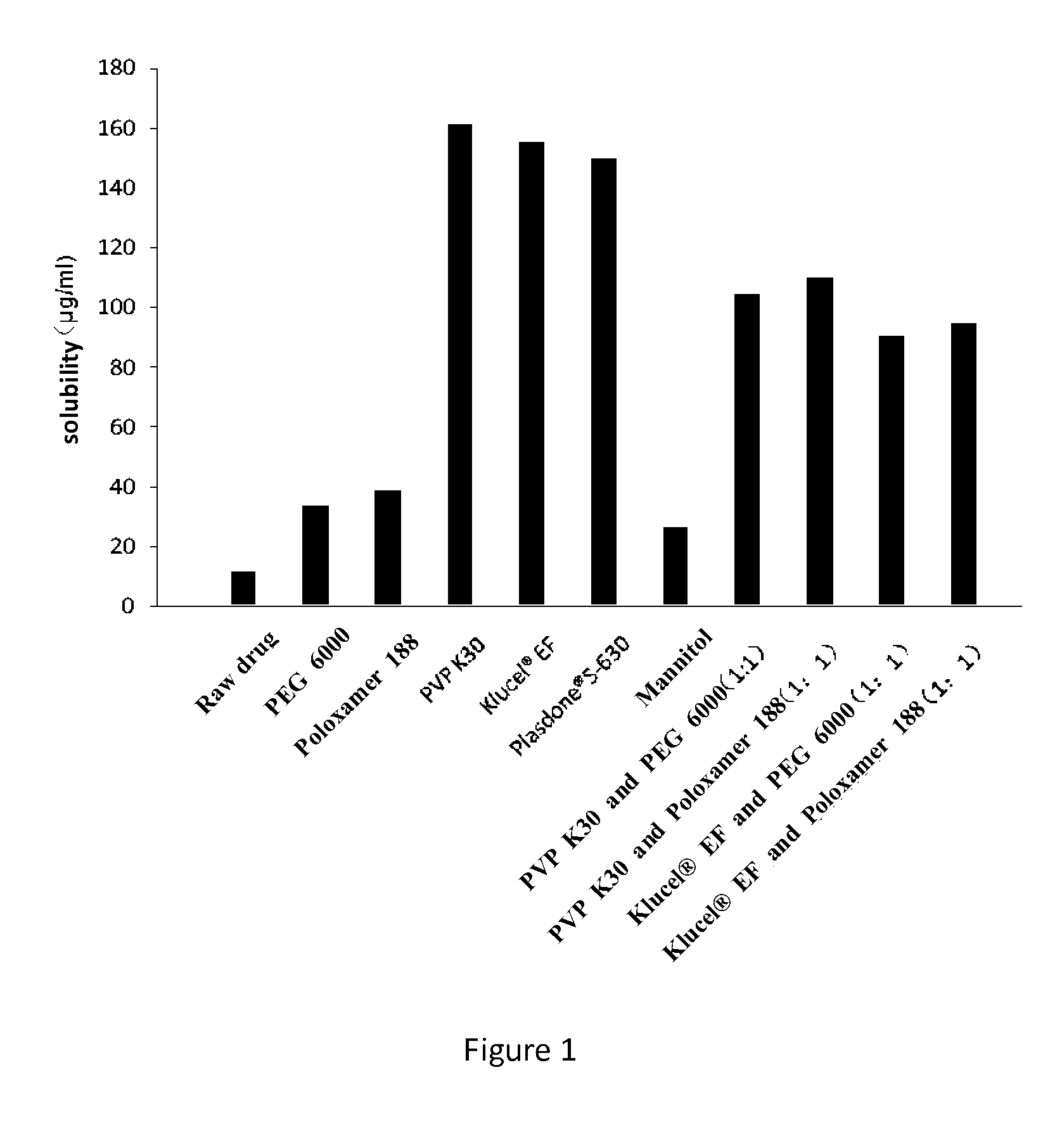
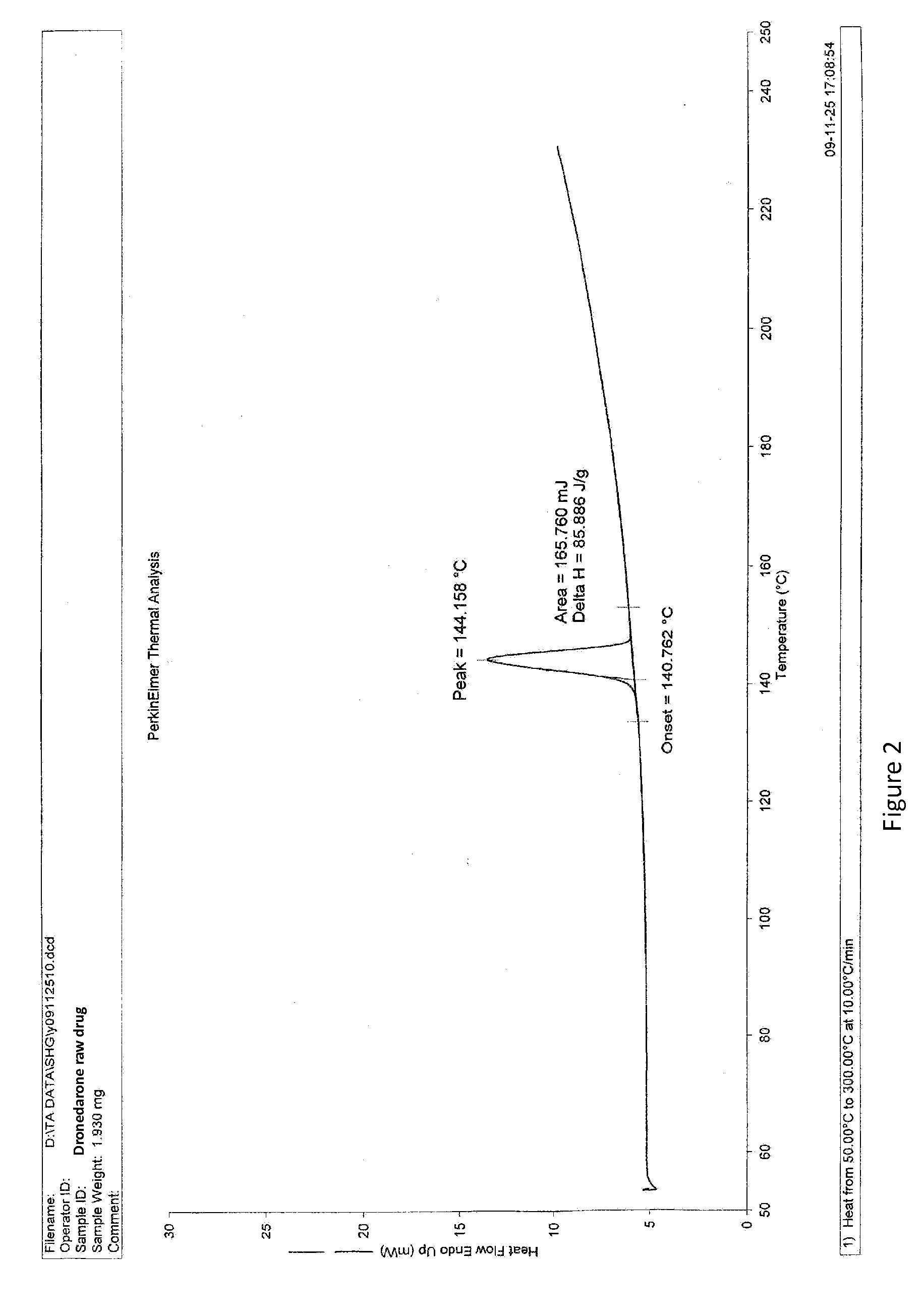
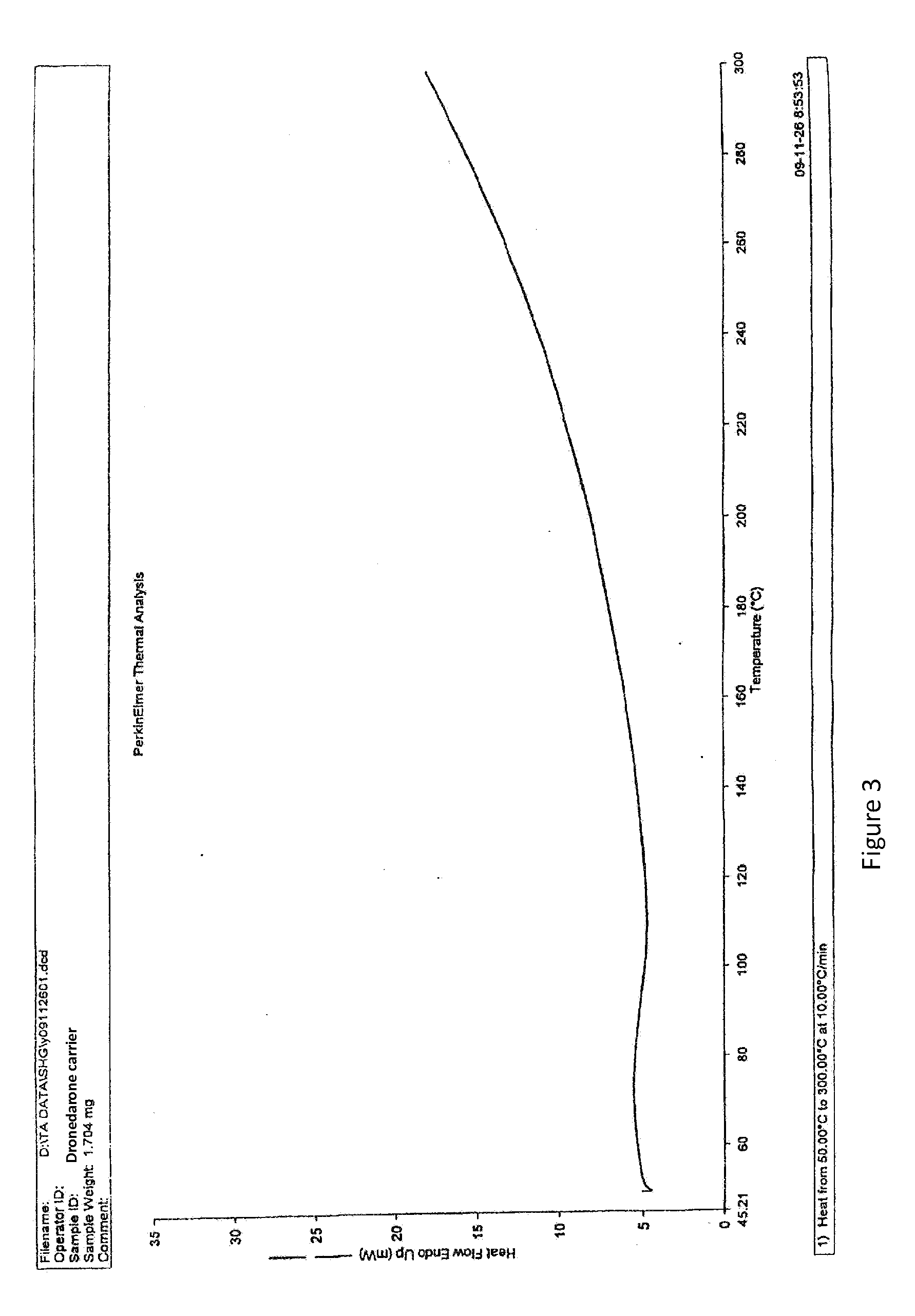
Dronedarone solid dispersion and preparation method thereof
a dronedarone and solid dispersion technology, applied in the direction of biocide, cardiovascular disorder, drug composition, etc., can solve the problems of low bioavailability, low and inability to achieve transmembrane absorption, etc., to achieve the effect of increasing the drug loading of the solid dispersion and improving the solubility or dissolution rate of dronedaron
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0048]One part by weight of dronedarone hydrochloride and 0.5 parts by weight of PVP K-30 were weighted and three parts by weight of dichloromethane were added into and the mixture was dissolved by stirring. Then the solution was transferred to a vacuum oven and dried under reduced pressure at 55° C. for 48 h. The dry mass was crushed and passed through a 100 mesh sieve thereby the solid dispersion of dronedarone hydrochloride was obtained.
example 2
[0049]One part by weight of dronedarone hydrochloride and 1.5 parts by weight of PVP K-30 were weighted and five parts by weight of dichloromethane were added into and the mixture was dissolved by stirring. Then the solution was transferred to a vacuum oven and dried under reduced pressure at 55° C. for 48 h. The dry mass was crushed and passed through a 100 mesh sieve thereby the solid dispersion of dronedarone hydrochloride was obtained.
example 3
[0050]One part by weight of dronedarone hydrochloride and three parts by weight of PVP K-30 were weighted and twelve parts by weight of dichloromethane were added into and the mixture was dissolved by stirring. Then the solution was transferred to a vacuum oven and dried under reduced pressure at 55° C. for 48 h. The dry mass was crushed and passed through a 100 mesh sieve thereby the solid dispersion of dronedarone hydrochloride was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight ratio | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com