Extraction of metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
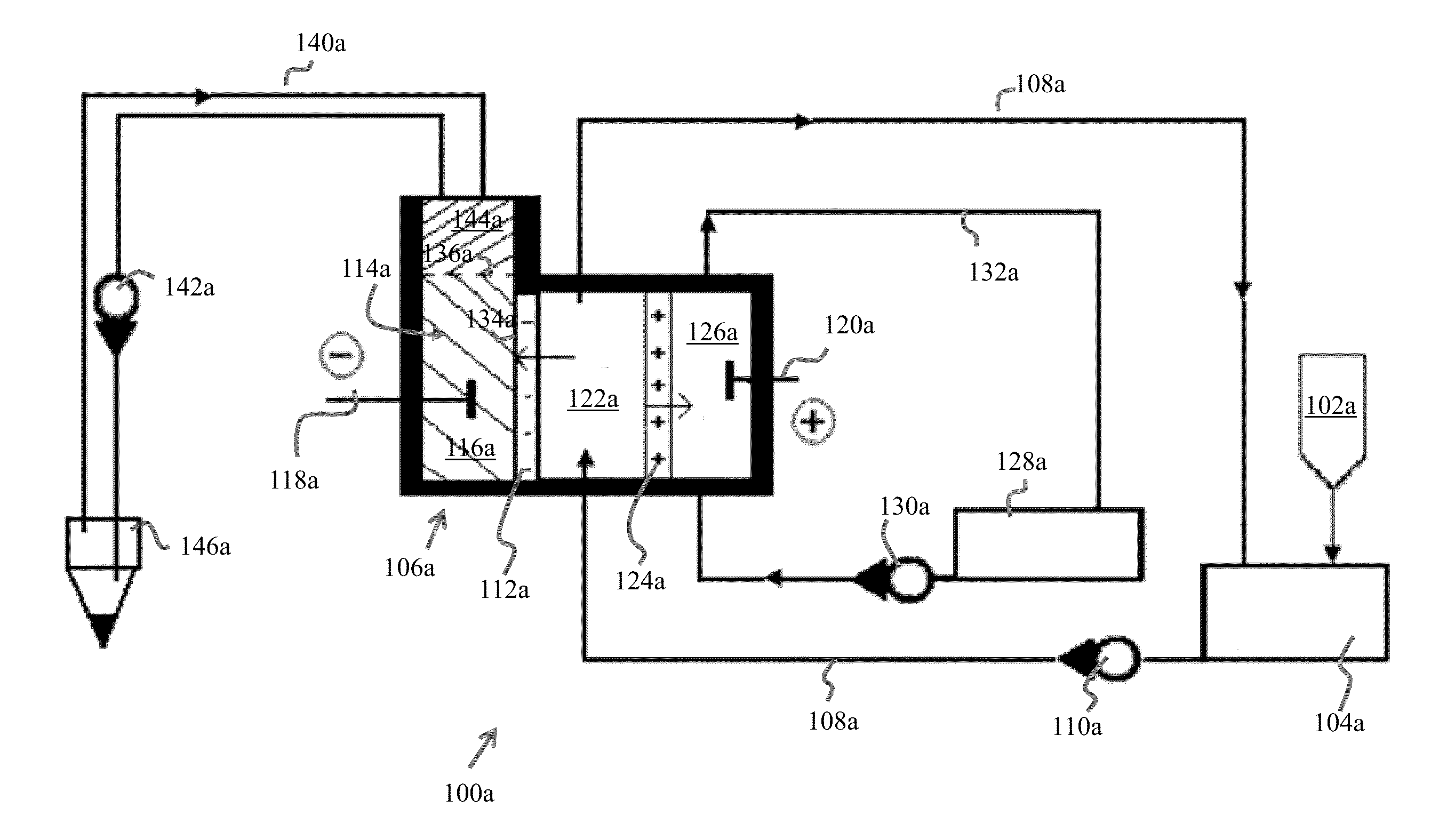
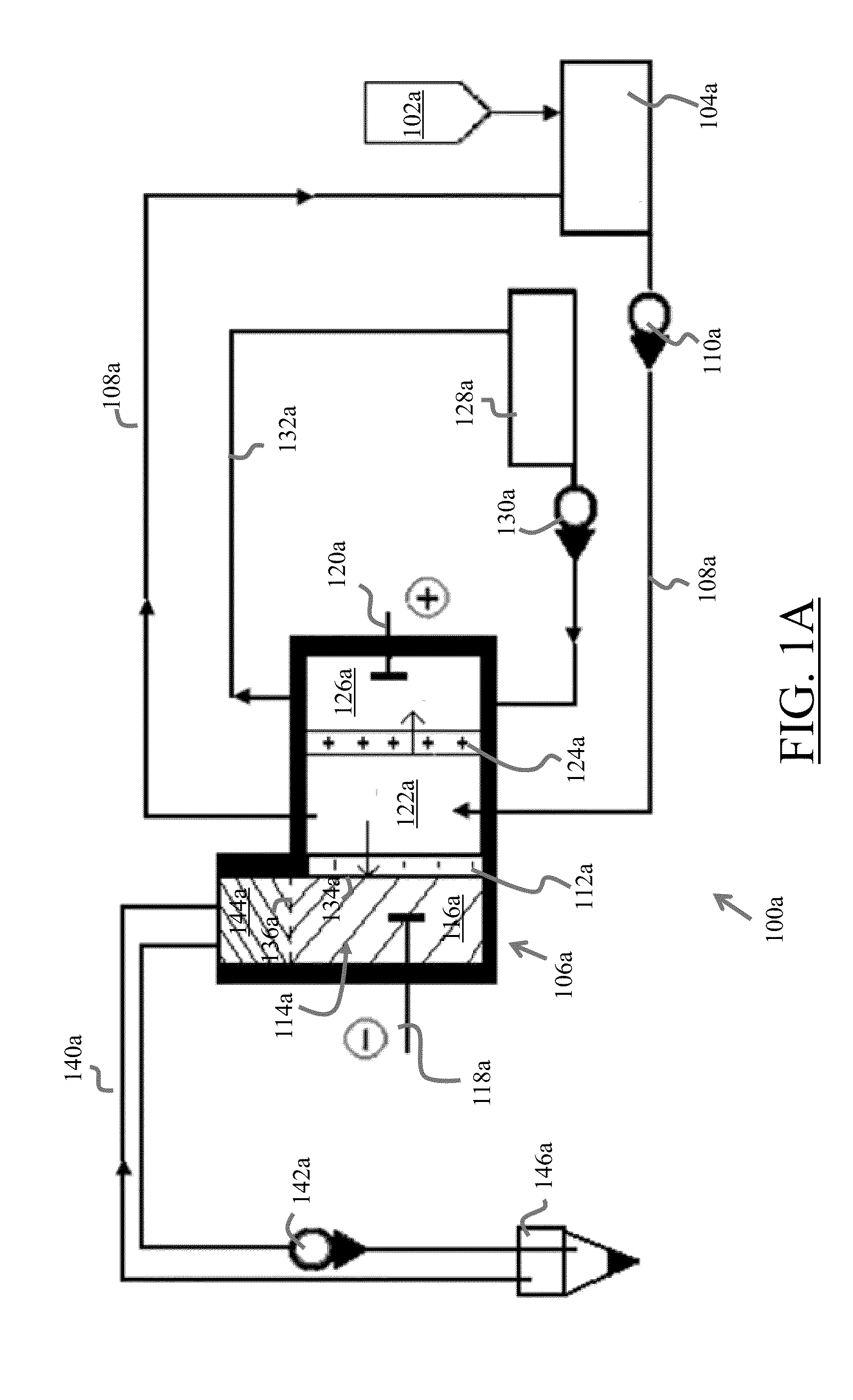
[0065]FIG. 1A illustrates the water tank of the unit where magnesium chloride water solution having 50 g / l concentration is filled.
[0066]Electro-dialyzer characteristics are as followings:
[0067]Voltage 10 V
[0068]Membrane types MK-40, MA-40, MB-1E
[0069]Membrane surface 100 cm2
[0070]Membranes numbers 3 (one membrane from each kind)
[0071]Cathode chamber volume 30 cm3
[0072]The distance between membranes 0.3 cm
[0073]Current density 0.03 A / cm2
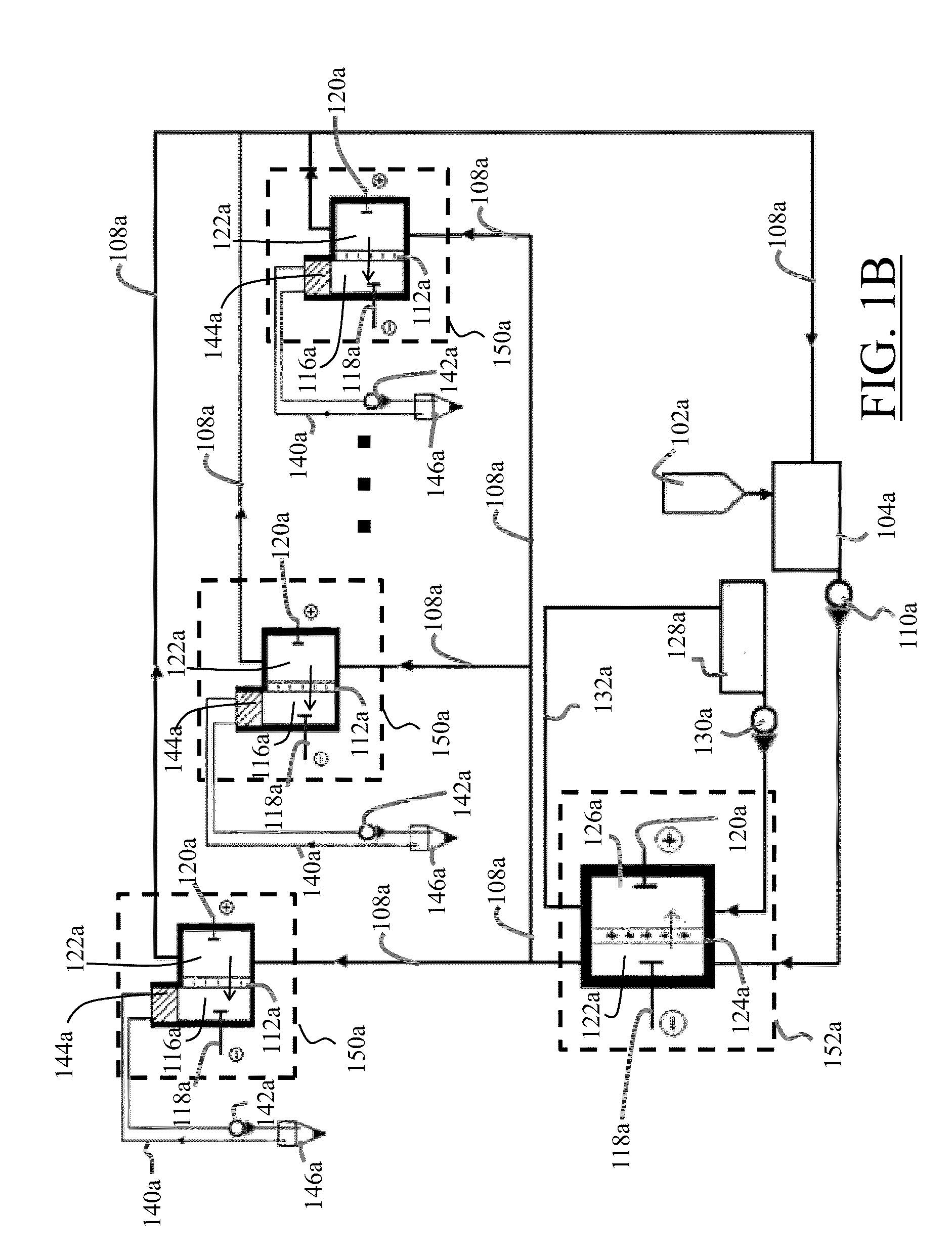
[0074]The cathode chamber 114 of the electro-dialyzer 106 is mercury filled (liquid metal 116), which serves as a cathode 116, while a stainless steel plate (120) serves as an anode 120. A removal medium layer 144 covers the cathode 116 and functions to remove the nano-particle metallic elements M from the top skin surface 136 of the cathode chamber 114 conductive liquid 116. The aqueous electrolyte solution is filled into the circulation tank 104 with the approximate, non-limiting exemplary rate of 1 liter / minute, from which it is pumped 110a and is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Electric charge | aaaaa | aaaaa |
| Current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com