Compositions and methods for treating myelofibrosis
a technology of myelofibrosis and compositions, applied in the direction of drug compositions, biocides, extracellular fluid disorders, etc., can solve the problems of insufficient mf management options, ineffective current treatment options, and high transplant-related morbidity and mortality,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
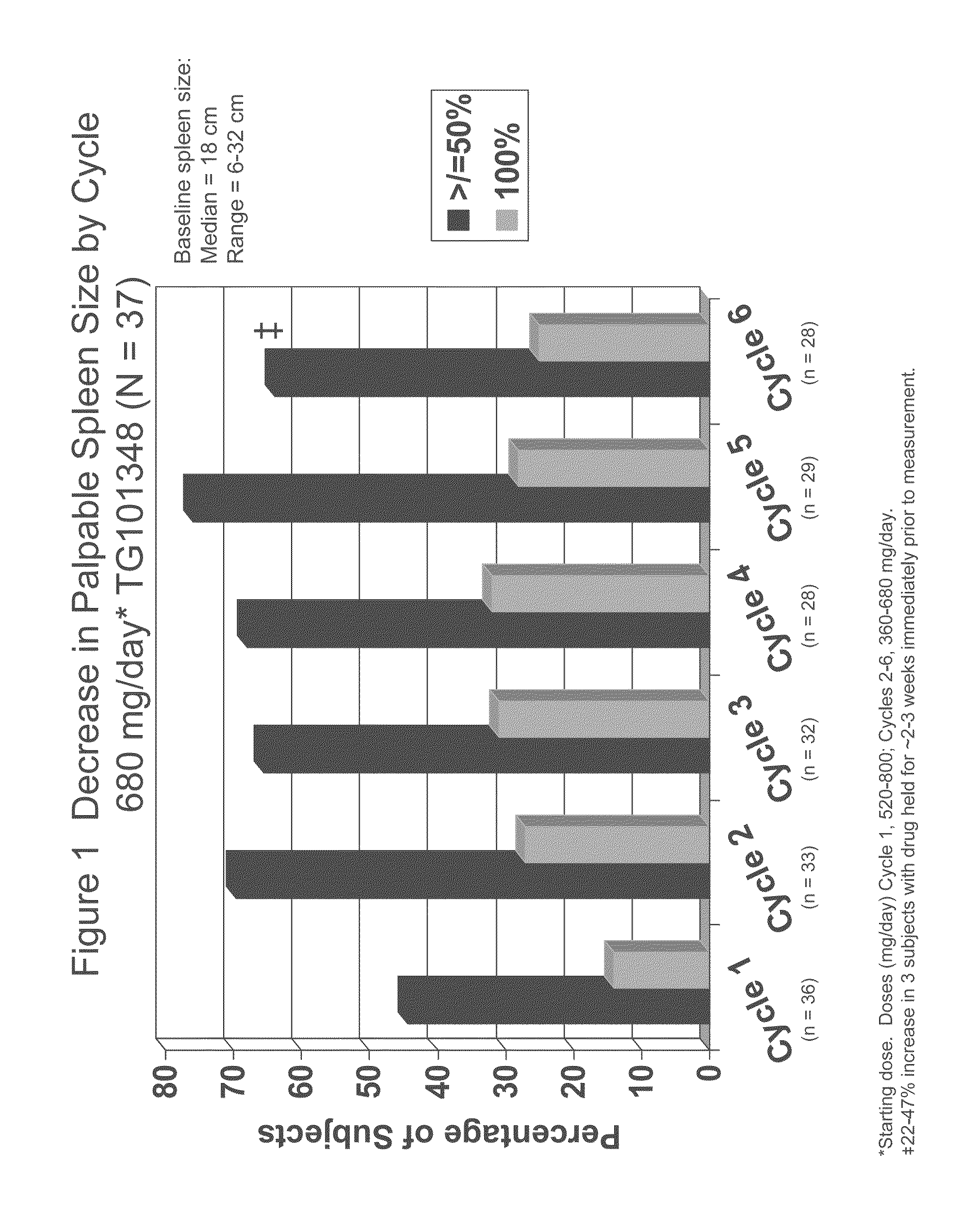
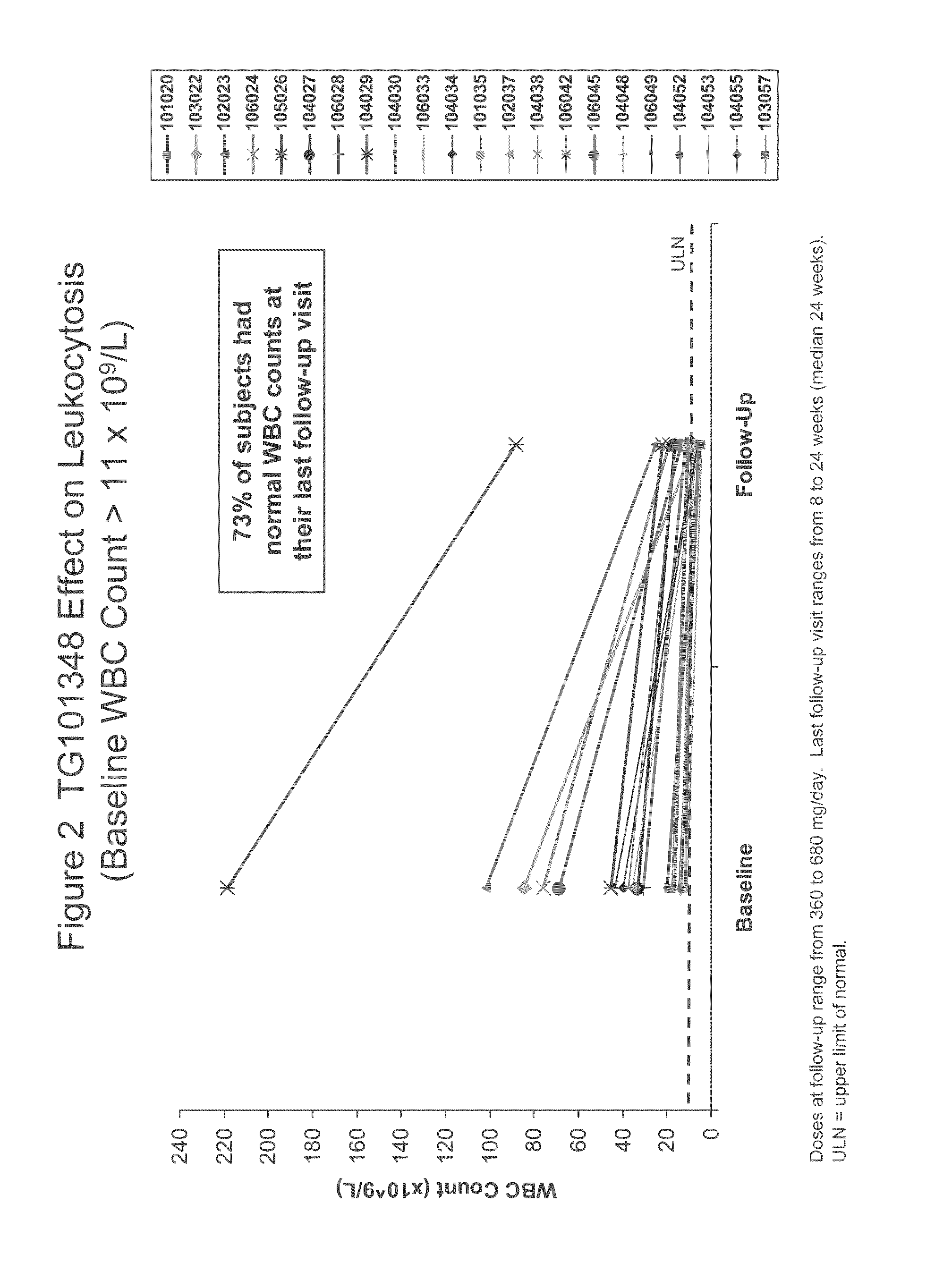
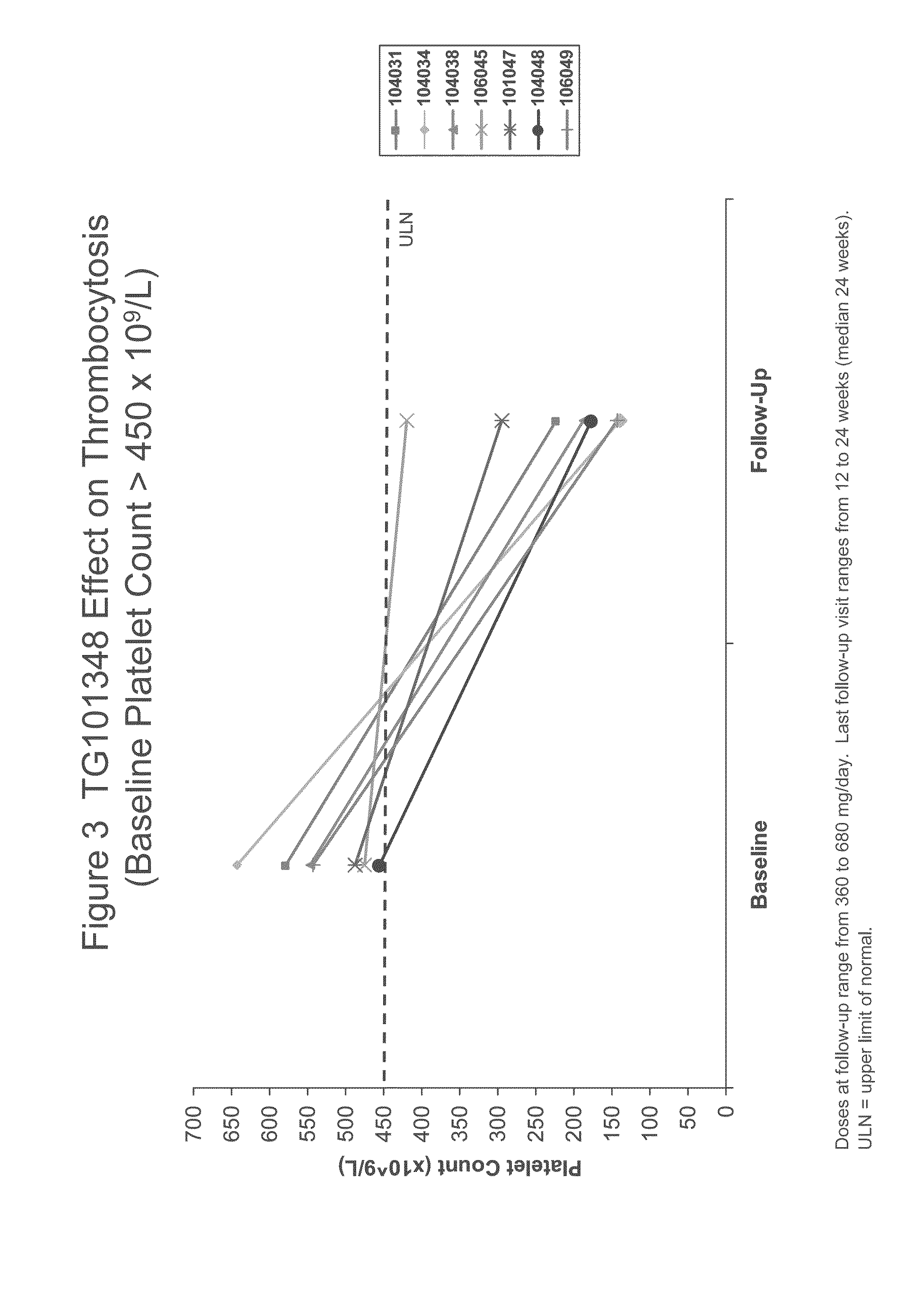
Evaluation of TG101348 in Myelofibrosis
[0083]As used herein, “TG101348” refers to N-tert-butyl-3-[(5-methyl-2-{[4-(2-pyrrolidin-1-ylethoxy)phenyl]amino}pyrimidin-4-yl)amino]benzenesulfonamide dihydrochloride monohydrate. The subjects in this study were administered with capsule form of TG101348 as described in Example 5. TG101348 was evaluated in a Phase I study for the treatment of myelofibrosis. This study was ongoing at the time the data were collected.
[0084]Background:
[0085]TG101348 is a potent, orally bioavailable, JAK2-selective small molecule inhibitor, that was evaluated in a Phase I study for the treatment of myelofibrosis. The dose-limiting toxicity was asymptomatic grade 3 or 4 amylasemia / lipasemia that was reversible, and the maximum tolerated dose (“MTD”) was 680 mg. The most frequent non-hematological toxicities were mild nausea, vomiting, and / or diarrhea that were easily controlled or resolved spontaneously. Grade 3 / 4 neutropenia and thrombocytopenia were observed in ...
example 2
Evaluation of TG101348 in Myelofibrosis
[0094]The subjects in this study were administered with capsule form of TG101348. TG101348 was evaluated in a Phase I study for the treatment of myelofibrosis. This study is also described in Example 1. This example describes data available at the time of data collection.
[0095]This study was an open-label, multicenter, and dose-escalation study with expanded cohort dose confirmation at MTD. The primary objective of this study was to determine safety / tolerability, DLT, MTD, and pharmacokinetics of TG101348 in subjects with MF. The secondary objective of this study was to evaluate preliminary clinical and pharmacodynamic activity.
[0096]The key eligibility criteria for subjects included: Myelofibrosis (PMF or post-PV / ET MF); High-risk or intermediate-risk with symptomatic splenomegaly / unresponsive to available therapy; ECOG performance status=2; ANC=1×109 / L; Platelet count=50×109 / L; Serum creatinine=2 mg / dL; Total bilirubin=2 mg / dL; AST / ALT=3× upp...
example 3
Evaluation of TG101348 in Myelofibrosis
[0108]The subjects in this study were administered with capsule form of TG101348.
[0109]Study Design:
[0110]The study constituted a Phase 1, dose-escalation trial (MF-TG101348-001). This study is also described in Examples 1 and 2. Study eligible patients were =18 years of age with high- or intermediate-risk primary myelofibrosis (PMF), post-PV MF, or post-ET MF (Tefferi A et al., Leukemia 22:14-22, 2008). Additional eligibility criteria and participating centers are listed in Table 6. All patients provided written informed consent. The primary endpoints were determination of safety and tolerability, dose-limiting toxicity (“DLT”), maximum tolerated dose (“MTD”) and pharmacokinetic (“PK”) behavior of TG101348. The secondary endpoint was assessment of therapeutic activity.
TABLE 6Detailed enrollment criteria for MF-TG101348-001Inclusion CriteriaExclusion Criteria1. Diagnosis of MF (PMF, post-PV MF, or post-1. Any chemotherapy, immunomodulatory drug...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com