Targeted protein silencing using chimeras between antibodies and ubiquitination enzymes
a technology of ubiquitination enzymes and chimeras, which is applied in the field of targeted protein silencing using chimeras between antibodies and ubiquitination enzymes, can solve the problems of curtailing the ubiquitination of both native and novel substrates, unable to provide phenotypic insight into cellular processes and disease etiology, and only able to study cellular characteristics by employing, etc., to achieve simple and efficient, simple implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Plasmids
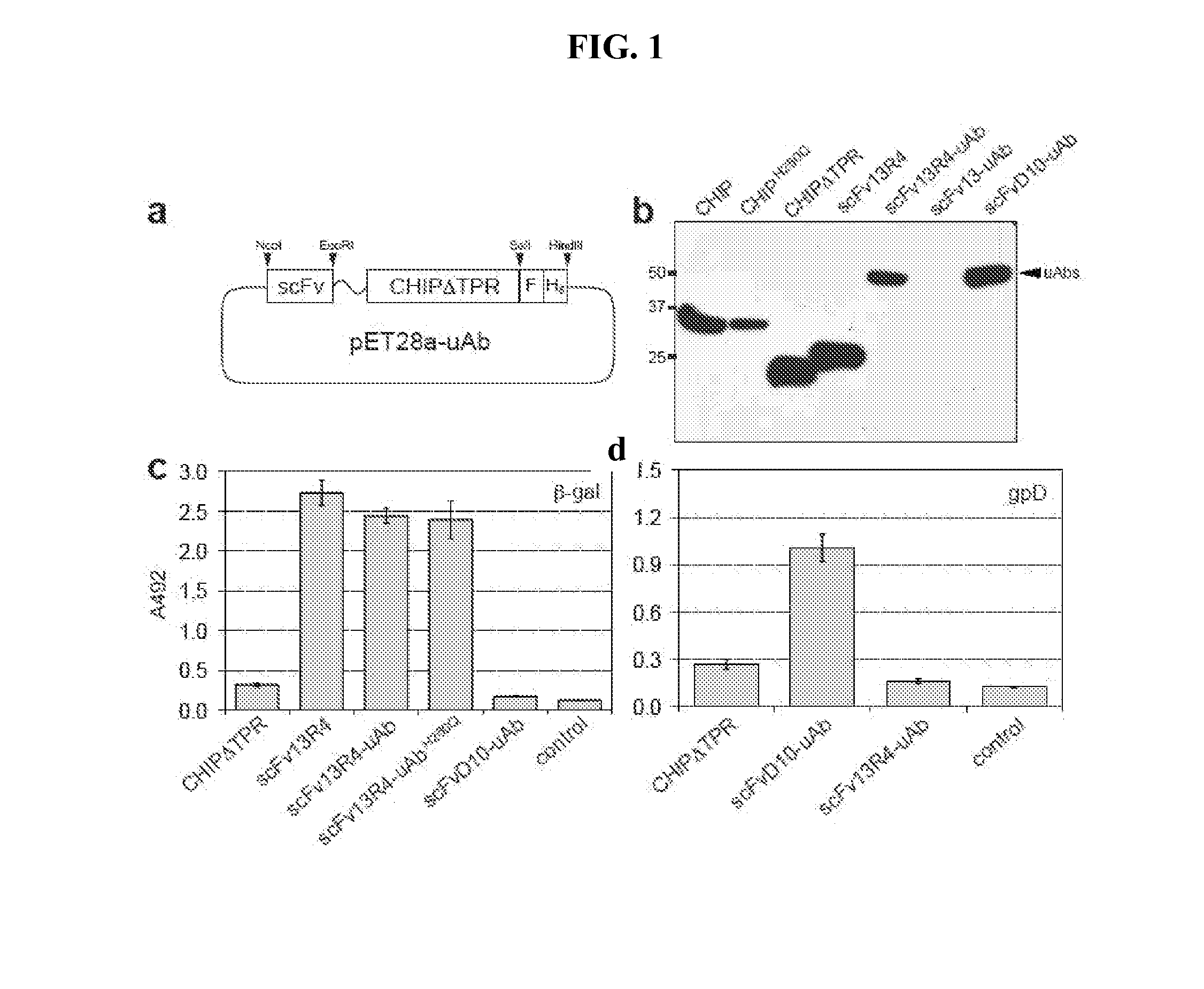
[0133]For prokaryotic expression of ubiquibodies, pET28a with C-terminal FLAG and His6 tags was used. CHIP and CHIPΔTPR were cloned from pET30a-hCHIP-His6. scFv13 and scFv13R4 were cloned from pET-scFv13(R4)-SecM17 vectors (see Contreras-Martinez, et al., “Intracellular Ribosome Display via SecM Translation Arrest as a Selection for Antibodies with Enhanced Cytosolic Stability.”J Mol Biol 372(2): 513-24 (2007)), which is hereby incorporated by reference in its entirety, while and scFvD10 was cloned from pHK49-gpD-specific antibody D10. See Koch et al., “Direct Selection of Antibodies from Complex Libraries with the Protein Fragment Complementation Assay.”J Mol Biol 357(2): 427-41 (2006), which is hereby incorporated by reference in its entirety. scFvs were cloned between NcoI and EcoRI sites, followed by a Gly-Ser-Gly-Ser-Gly (SEQ ID NO: 1) linker that was inserted by PCR before CHIPΔTPR followed by a SalI site. The FLAG and His6 tags were created by primer dimerization and ...
example 2
Antibodies and Reagents
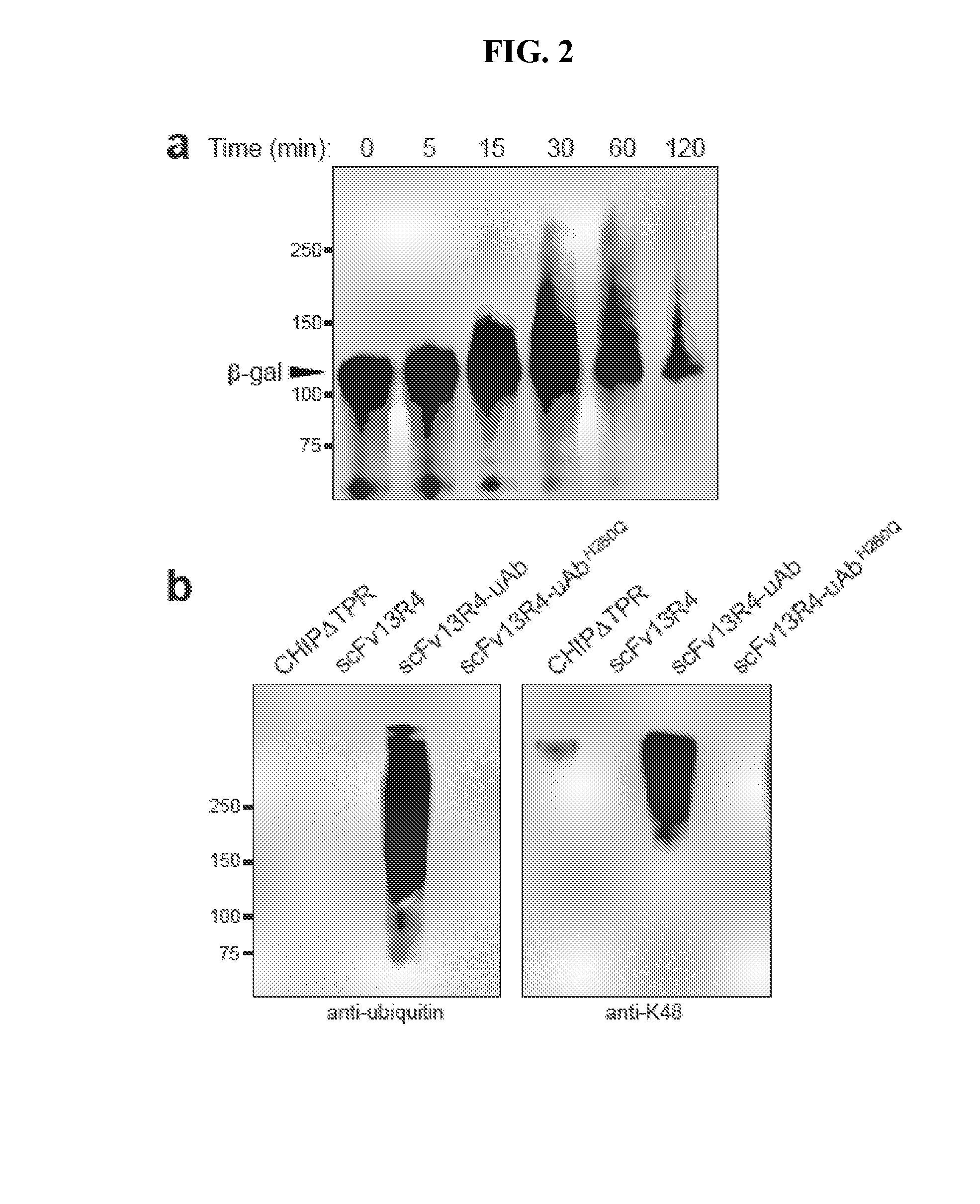
[0135]Mouse monoclonal [M2] to DDDDK tag (HRP) from Abcam (ab49763) was used to probe for ubiquibodies while rabbit anti-HA produced by Sigma (H6908) was used to probe the antigen gpD and rabbit polyclonal anti-β-gal from Abcam (ab616) was used to probe the antigen β-gal. Rabbit polyclonal anti-ubiquitin from Abcam (ab8134) and rabbit anti-ubiquitin Lys48-specific from Millipore (clone Apu2) were used to detect poly-ubiquitin chains. Mouse monoclonal anti-HSP70 from Enzo Life Sciences (C92F3A-5) and mouse monoclonal anti-GFP from Roche were used as controls for the eukaryotic expression. All of the above were used with secondary antibodies from Promega, anti-mouse-HRP and anti-rabbit-HRP.
example 3
In Vitro Reconstitution Assays
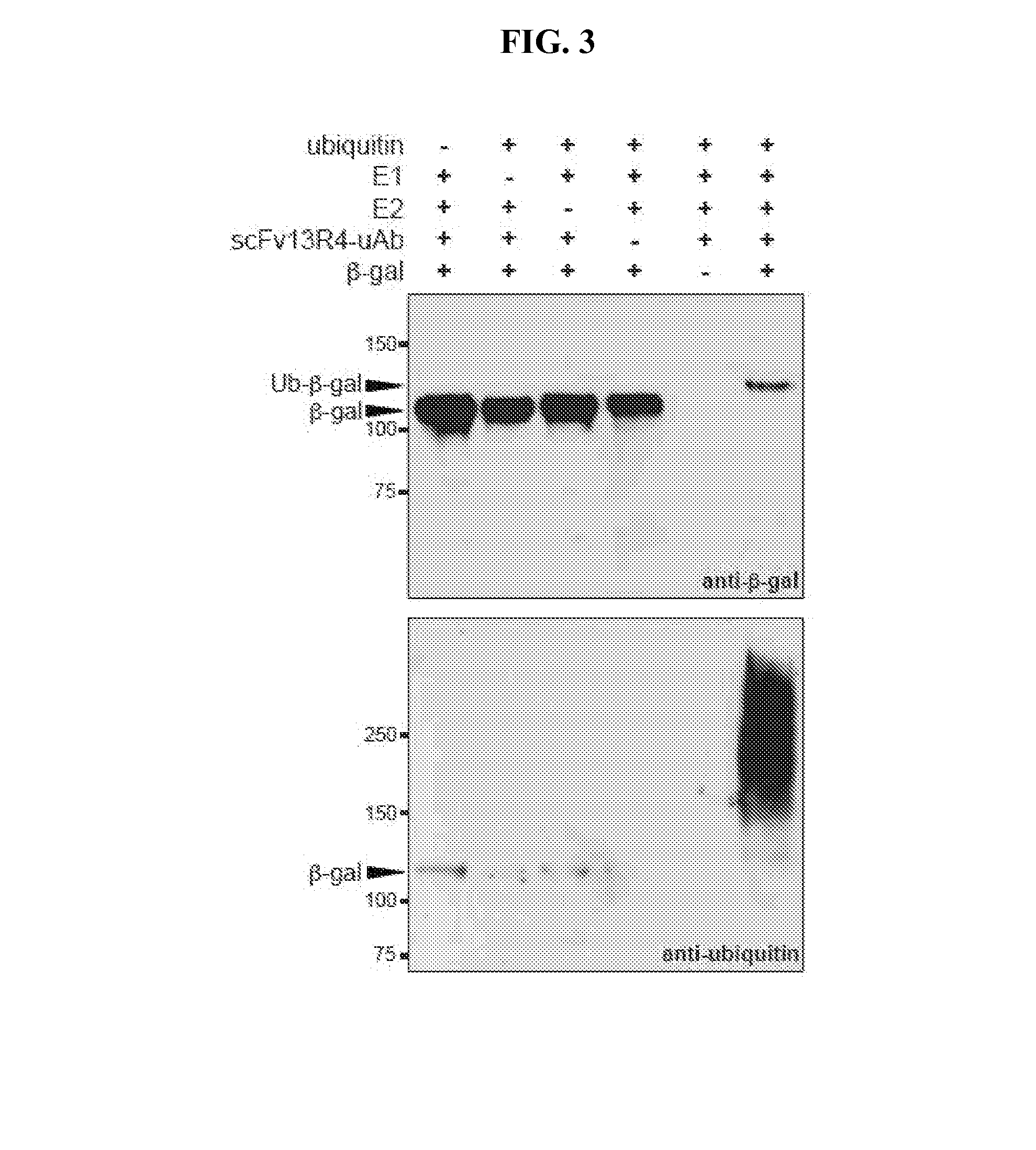
[0136]Purified ubiquitin and ubiquitin activating enzyme (E1) were purchased from Boston Biochem, ubiquitin conjugating enzyme (E2, UbCH5a) was purchased from Millipore, Hsp70 was purchased from Enzo Life Sciences and β-gal was purchased from Sigma. Moreover, in vitro ubiquitination assays were performed in the presence of 20 mM MOPS pH 7.2, 100 mM KCl, 5 mM MgCl2 1 mM DTT, 4 mM ATP, 50 μM ubiquitin, 0.1 μM E1, 2 μM E2, 3 μM E3 (ubiquibody or control protein) and 3 μM target protein (β-gal, gpD or Hsp70). Reactions were carried out at 37° C. and analyzed by SDS-PAGE and immunoblotting.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com