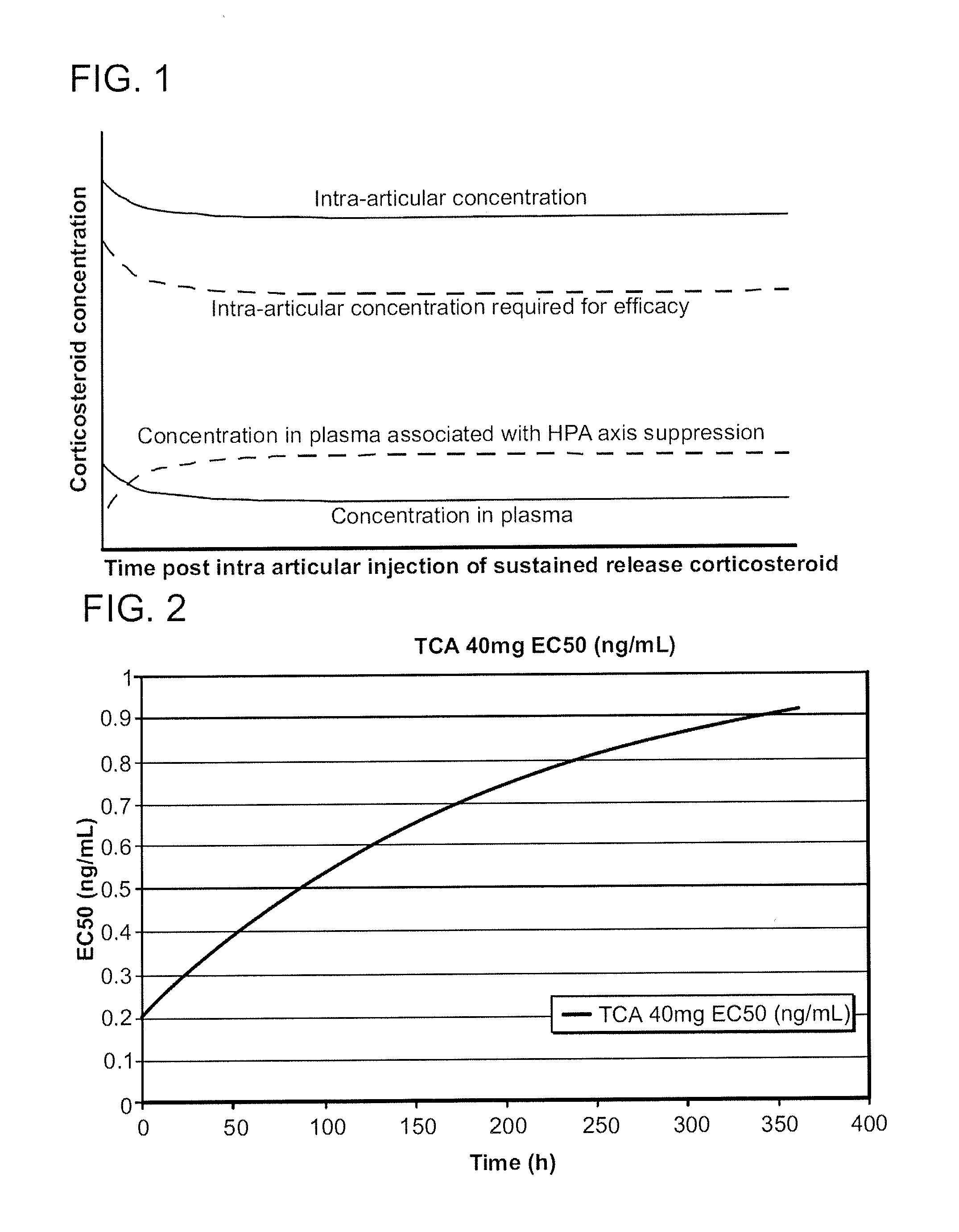
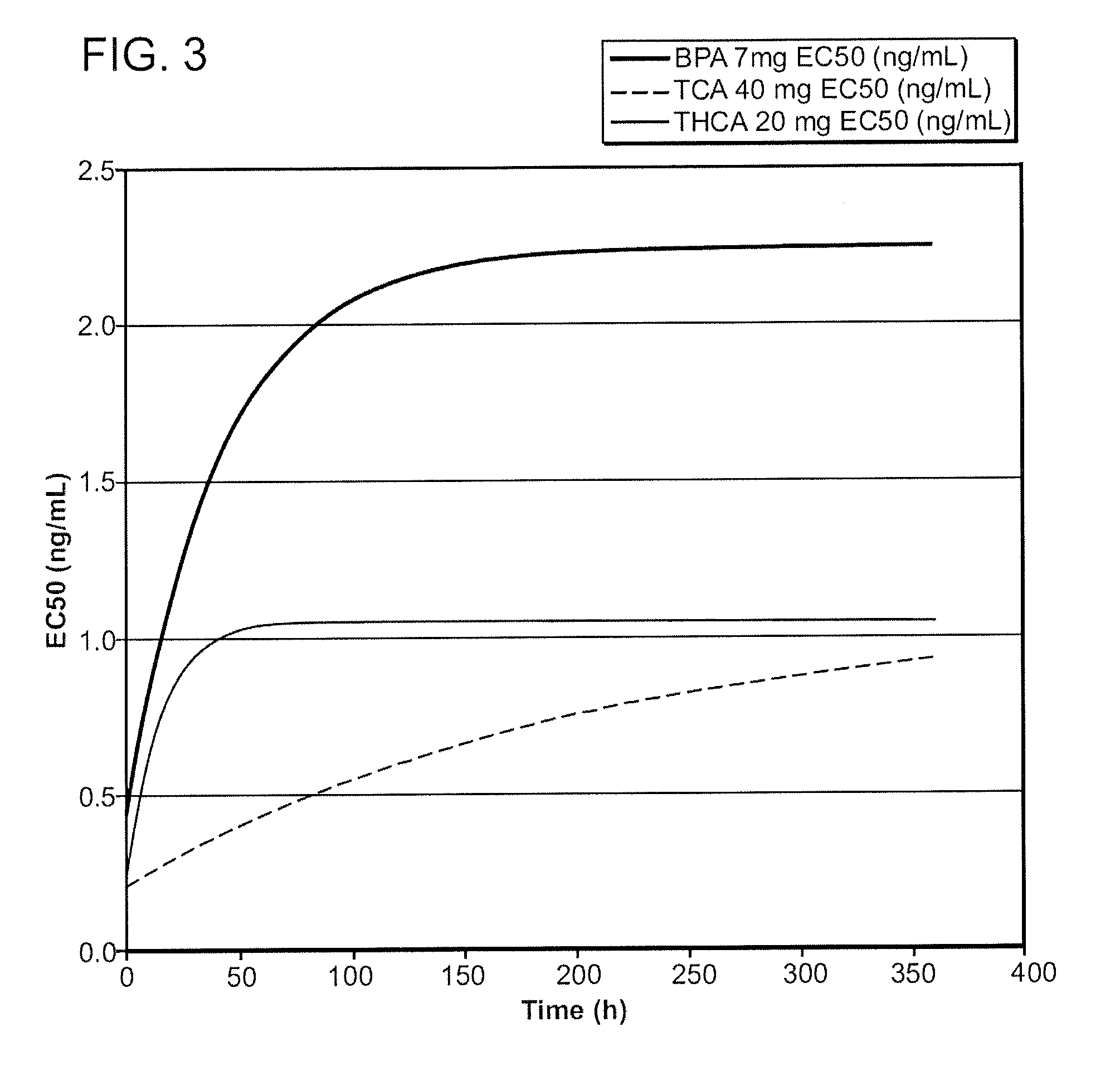
Corticosteroids for the Treatment of Joint Pain
a technology of corticosteroids and joint pain, which is applied in the direction of drug compositions, immune disorders, nervous disorders, etc., can solve the problems of affecting the treatment effect of pain and/or inflammation, affecting the treatment effect, so as to reduce pain and/or inflammation, reduce the effect of long-term side effects and minimal side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sustained-Release Betamethasone or Triamcinolone Acetonide Microparticles
[0224]In one embodiment, the microparticle formulation contains a copolymer of DL-lactide (or L-lactide) and glycolide in a 45:55 molar ratio (up to 75:25 molar ratio) with an inherent viscosity ranging from 0.15 to 0.60 dL / g with either an ester or acid end group plus either the corticosteroid betamethasone or triamcinolone acetonide. If betamethasone is used, then the betamethasone is in the form of either betamethasone acetate, betamethasone diproprionate or a combination thereof. The total amount of betamethasone or triamcinolone acetonide incorporated into the microparticle ranges from 10% to 30% (w / w). The microparticles are formulated to mean mass range in size from 10 to 100 microns. The population of microparticles is formulated to be delivered through a 19 gauge or higher needle. Additional excipients may be added such as, but not limited to, carboxymethylcellulose sodium, mannitol, polysorbate-80, so...
example 2
Sustained-Release Betamethasone or Triamcinolone Acetonide Microparticles with an Immediate Release Form
[0225]In another embodiment, the microparticle formulation of Example 1 is further admixed with an immediate release betamethasone or triamcinolone acetonide component, such as a betamethasone or triamcinolone acetonide containing solution. If betamethasone is used, then the betamethasone in the immediate release component is in the form of either betamethasone acetate, betamethasone diproprionate or a combination thereof. If betamethasone is used, then the immediate release component provides an initial release of a total of about 5 to 20 mg of betamethasone over the first 1-10 days, while the sustained release component releases betamethasone at a rate of about 0.1 to 1.0 mg / day over the first 14 to 90 days following administration. If triamcinolone acetonide is used, then the immediate release component provides an initial release of a total of 10 to 40 mg of drug over the firs...
example 3
Determination of Time-Variance in HPA Axis Sensitivity
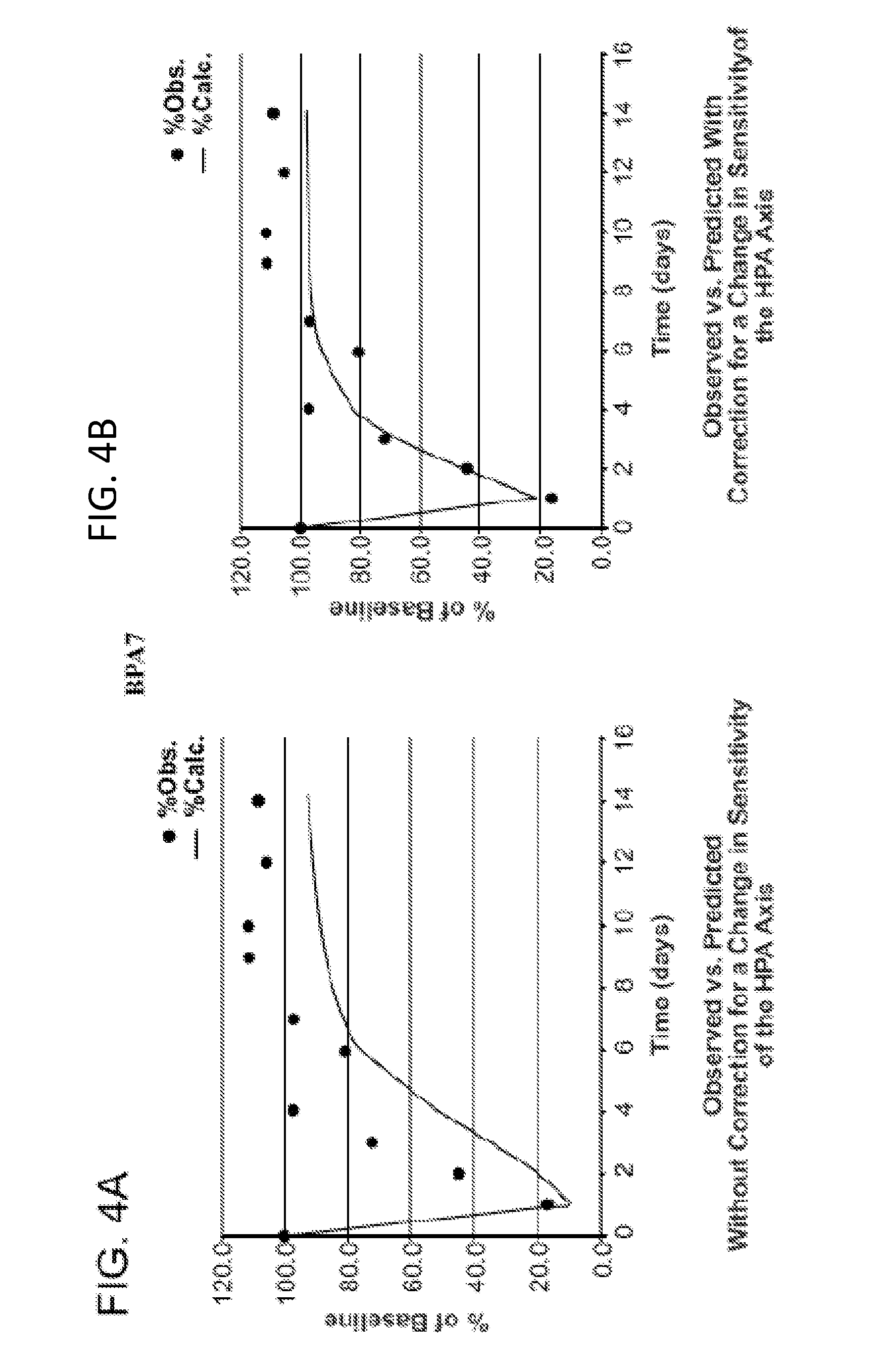
[0226]Adult volunteers (N=4 to 9 per group) give appropriate informed consent. Each individual in each group receives a single intra-articular administration of an exogenous corticosteroid (triamcinolone acetonide 40 mg; triamcinolone hexacetonide 20; betamethasone 7 mg (disodium phosphate 4 mg / acetate 3 mg). Blood samples for measurement of corticosteroid concentrations and / or cortisol concentrations are drawn at 8 AM at baseline and on days 1, 7, 9, 10, 12, 14, 18, and 21. The extent of suppression of endogenous cortisol was measured in each subject in each group. The extent of cortisol suppression predicted by previously published models (Meibohm, 1999) was determined and compared to observations (FIGS. 4A, 4C and 4E). The change (decrease) in HPA axis sensitivity vs. time is then determined on a day-by-day and final basis (FIGS. 4B, 4D and 4F), permitting determination of the correct steady-state intra-articular doses of cort...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com