Method and apparatus for chromatographic purification
a chromatographic and purification method technology, applied in the field of biotechnology and pharmaceutical industry, can solve the problems of difficult removal of subcellular fragments, high cost of affinity chromatography, and formidable challenge of human use as a therapeutic agen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0257]The following examples represent practical applications of the invention.
1.
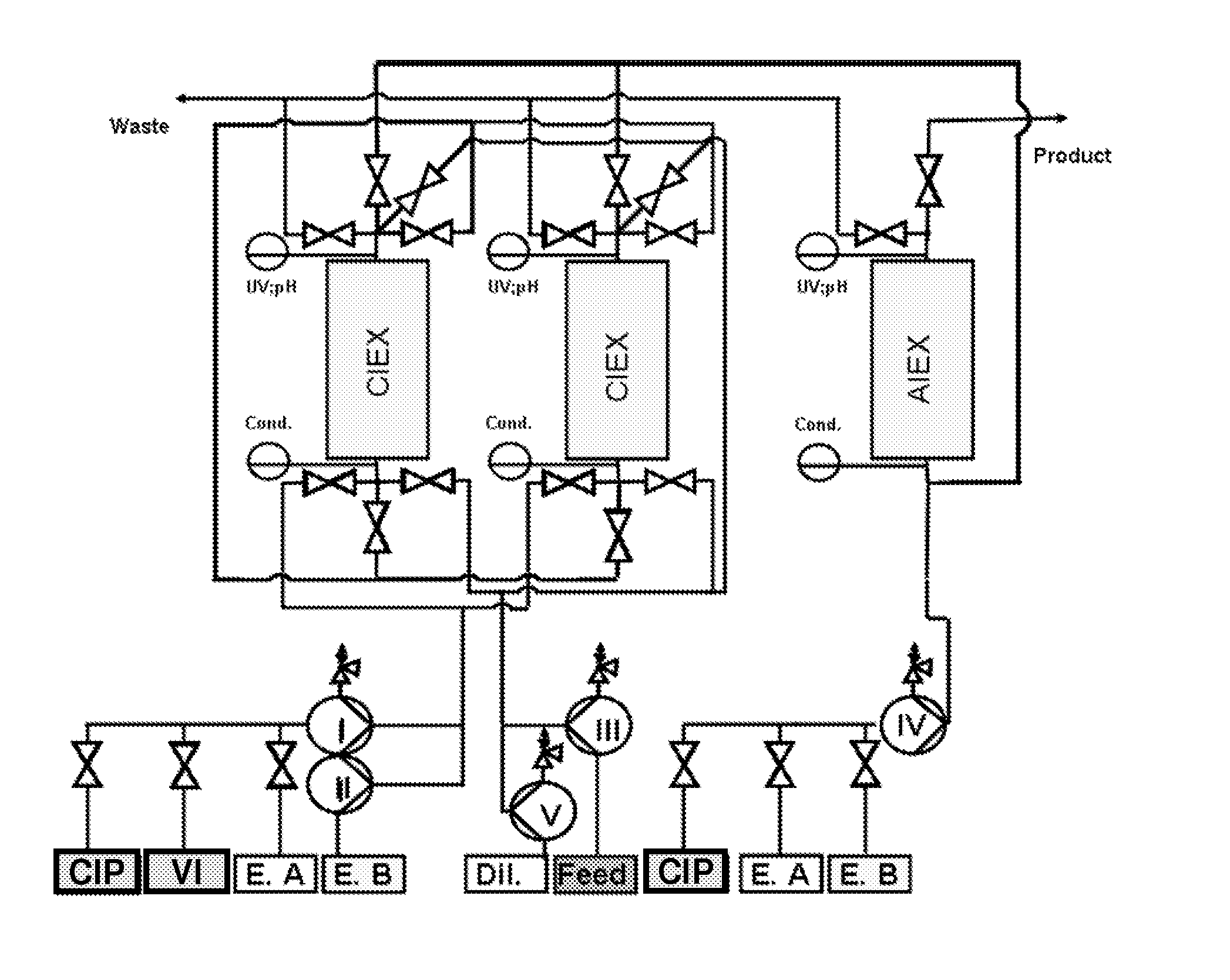
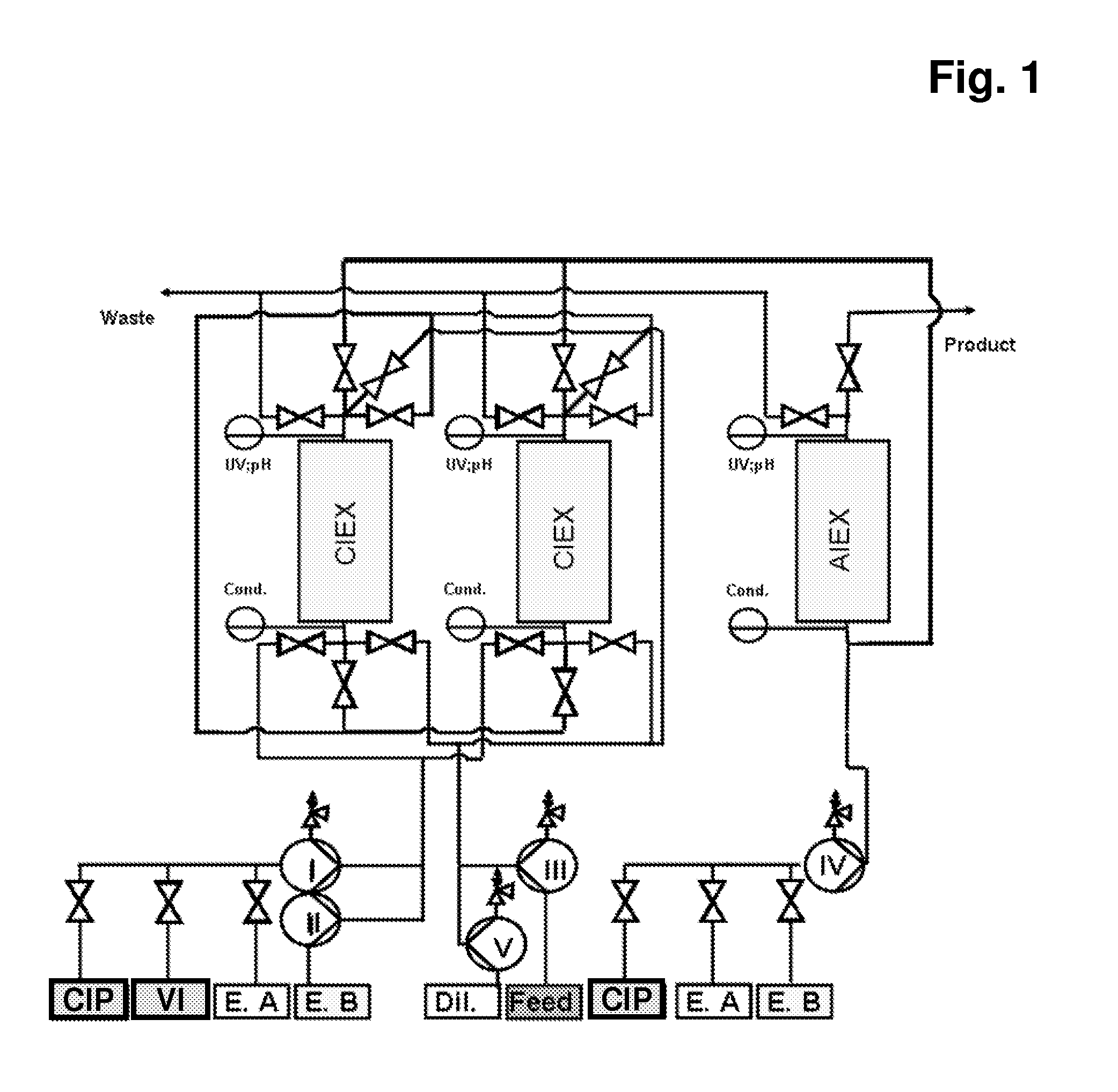
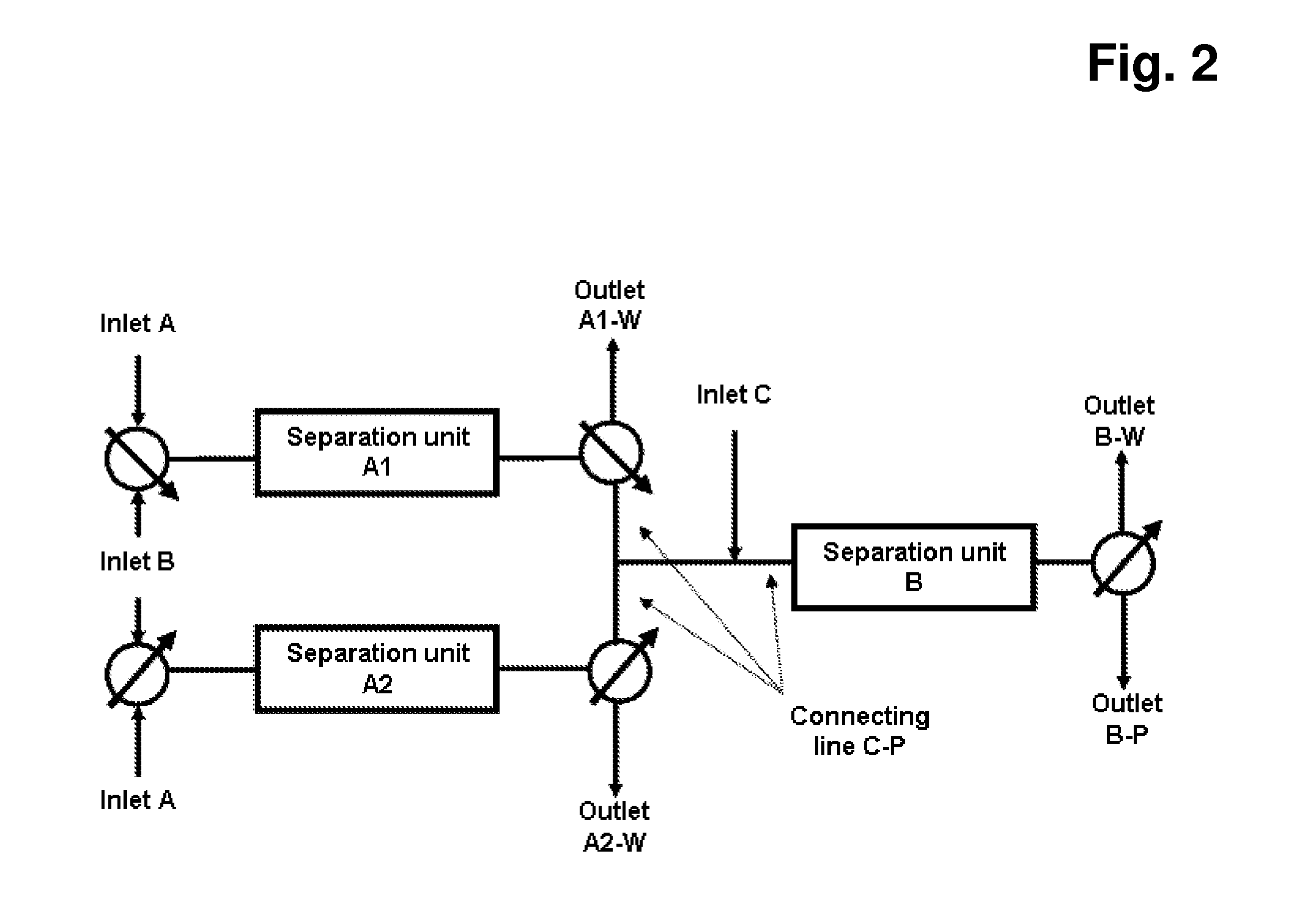
[0258]The monoclonal antibody cell culture solution, which had 0.9 mg / ml monoclonal antibody composing a fraction of 17% of all components in the solution (according to analytical SEC), where HCP amount was 600000 ng / mg antibody (according to immunoenzymetric assay SP 2 / 0), was purified on the Eshmuno™ S resin in first mode (resembling columns A1 and A2) and Capto™ Adhere in the second mode (resembling column B) under the following conditions. Chromatography conditions: first mode—33.5 ml Eshmuno™ S resin was packed in a 16×150 mm column; the column was then equilibrated with 25 mM phosphate buffer containing 20 mM NaCl, pH 4.5 at 30 ml / min (1000 cm / hr). Second mode—33.5 ml Capto™ Adhere resin was packed in a 16×150 mm column; the column was then equilibrated with 50 mM TRIS buffer containing 20 mM NaCl, pH 9 at 15 ml / min (500 cm / hr). To prepare the sample: monoclonal antibody cell culture solution was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com