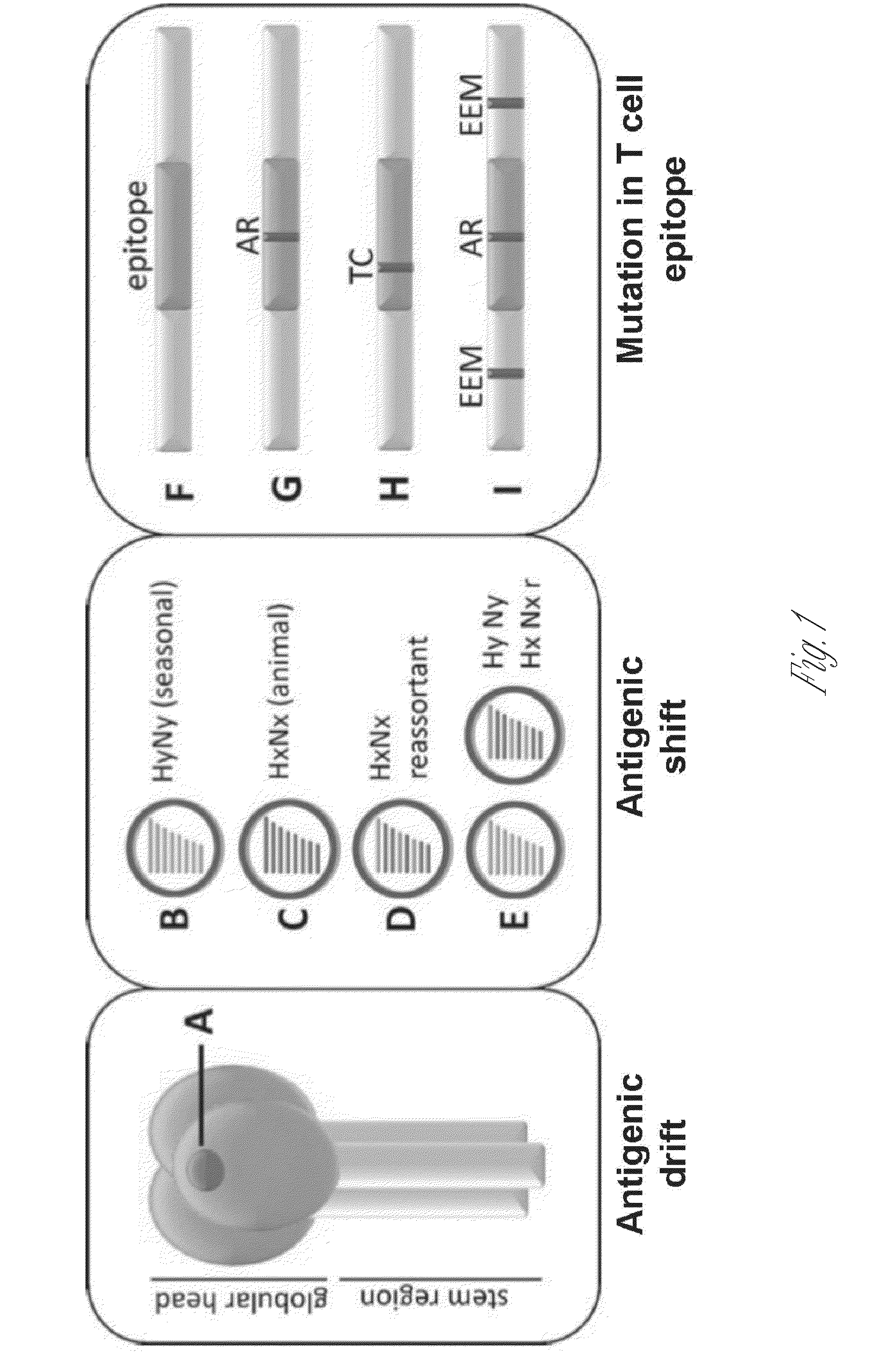
Broadly reactive mosaic peptide for influenza vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Materials and Methods
Cells and Viruses
[0108]Chicken embryo fibroblasts (CEFs) and Mardin-Darby canine kidney (MDCK) cells were obtained from Charles River Laboratories, Inc. (Wilmington, Wash.) and the American Type Culture Collection (ATCC, Manassas, Va.), respectively. Cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics. CEFs were used for propagating MVA virus. Highly pathogenic avian influenza (H5N1) virus A / Vietnam / 1203 / 04 was kindly provided by Dr. Yoshihiro Kawaoka (University of Wisconsin-Madison, Wis., USA). Highly pathogenic avian influenza (H5N1) viruses, A / Hongkong / 483 / 97, A / Mongolia / Whooper swan / 244 / 05 and A / Egypt / 1 / 08 and seasonal influenza viruses including A / Puerto Rico / 8 / 34 (PR8, H1N1) and A / Aichi / 2 / 1968 (H3N2) were kindly provided by Dr. Stacey Schultz-Chemy and Dr. Ghazi Kayali (St. Jude children's research hospital, Memphis, Term.). All viruses were propagated and titrated in MDCK cells w...
example ii
[0134]Samples were collected for a microneutralization test at 4 weeks post-immunization. FIG. 7 shows the antibody titers for three different strains of influenza virus in mice immunized with H5M / MVA, inactivated vikeus (Baxter) or MVA alone. The antisera in immunized mice were reactive against three distinct viral clades (HPAI Clade 0, Clade 1, and Clade 2 viruses) after a single dose. The presence of neutralizing antibodies at 4 weeks indicates that the H5M vaccine provides rapid immunity and the immunity is higher than inactivated against the homologous virus. Even recent consensus approaches that have tried to control for the most diversity of input sequence have failed to simultaneously elicit immune responses against all of these clades.
TABLE 1GrpConstructsRouteN =Challenge stains1MVA-H5MID8HK / 483 / 972Inactivated H5N1SC8HK / 483 / 973MVA / LUC - controlID5HK / 483 / 974MVA-H5MID8MONG / 244 / 055Inactivated H5N1SC8MONG / 244 / 056MVA / LUC - controlID5MONG / 244 / 057MVA-H5MID8VN / 1203 / 048Inactivated H...
example 111
[0136]The genetic algorithm was used to generate other mosaic sequences. 4,809 H1 sequences were used to generate 4 H1M sequences, e.g., using default parameters or modified parameters such as a different random seed number. A characteristic residue in one set of those sequences (SEQ ID Nos. 2 and 3) may be at position 125 (Ile), and a characteristic residue in the other set (SEQ ID Nos. 4 and 5) may be at one or more of positions 62 (Lys), 64 (Ile), 68 (Gln), 71 (Asn), 73 (Ser), 74 (Val), 86 (Leu), 88-91 (IleSerLysGlu), 99-103 (LysProAsnProGlu), 111 (His), or 113 (Ala), or corresponding positions (depending on the length of the signal peptide).
[0137]2,931 H3 sequences were used to generate a H3M sequence (SEQ ID NO:7).
[0138]393 H2 sequences were used to generate a H2M sequence (SEQ ID NO:6). A characteristic residue in H2M may be at one or more of positions 24 (Ala), 45 (Lys), 86 (Ser), 258 (Thr), 260 (Asn), or 261 (Leu), or corresponding positions.
[0139]799 H7 sequences were used ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com