Point of care immunization testing system
a testing system and immunization technology, applied in the field of point of care immunization testing system, can solve the problems of not having a point of care device that can quickly and inexpensively requiring more than a week for response time at high “per test” cost, and unable to quickly diagnose the immunity level of patients, etc., to achieve convenient sample transfer, improve assay performance, and facilitate the sample preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
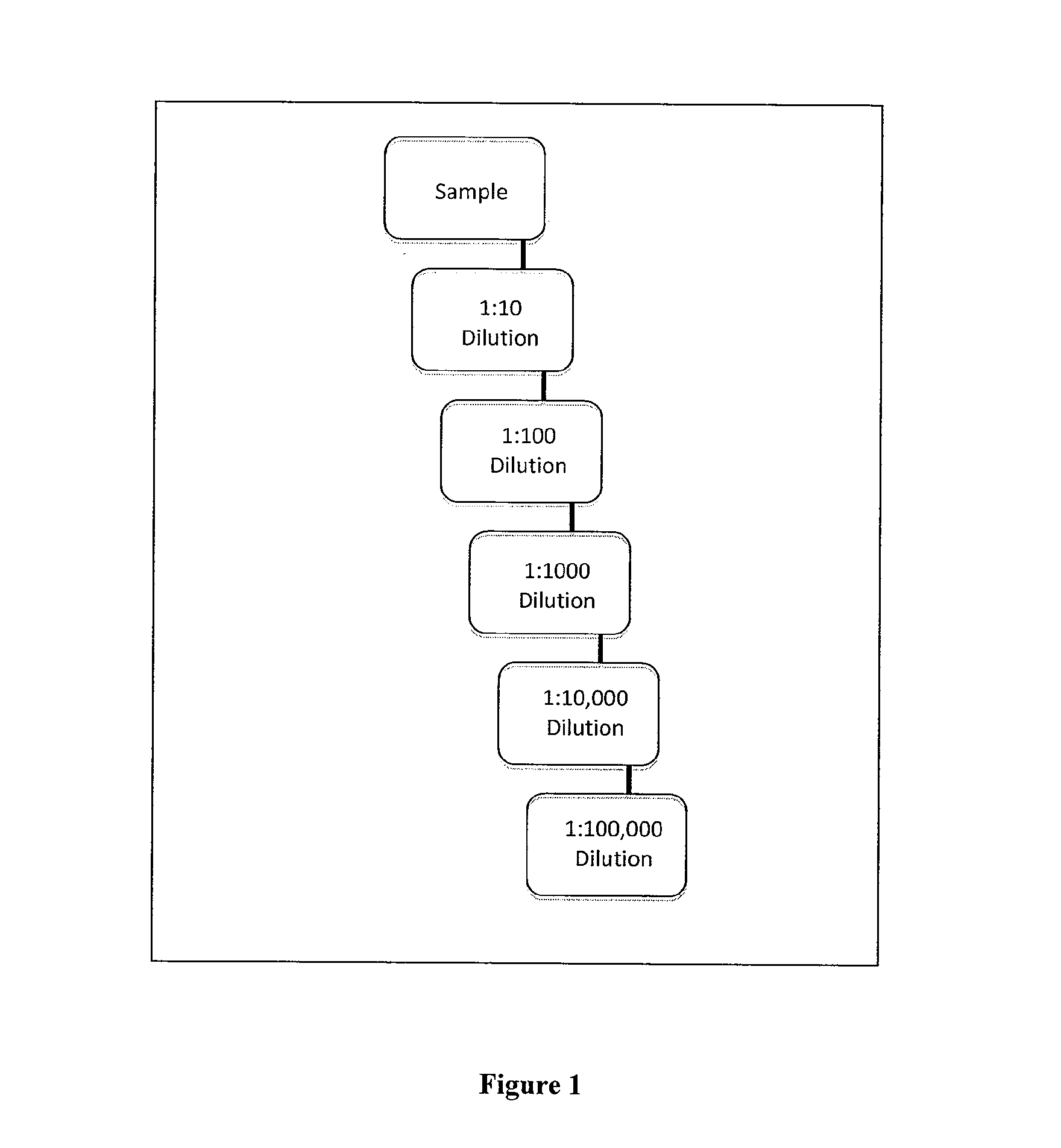
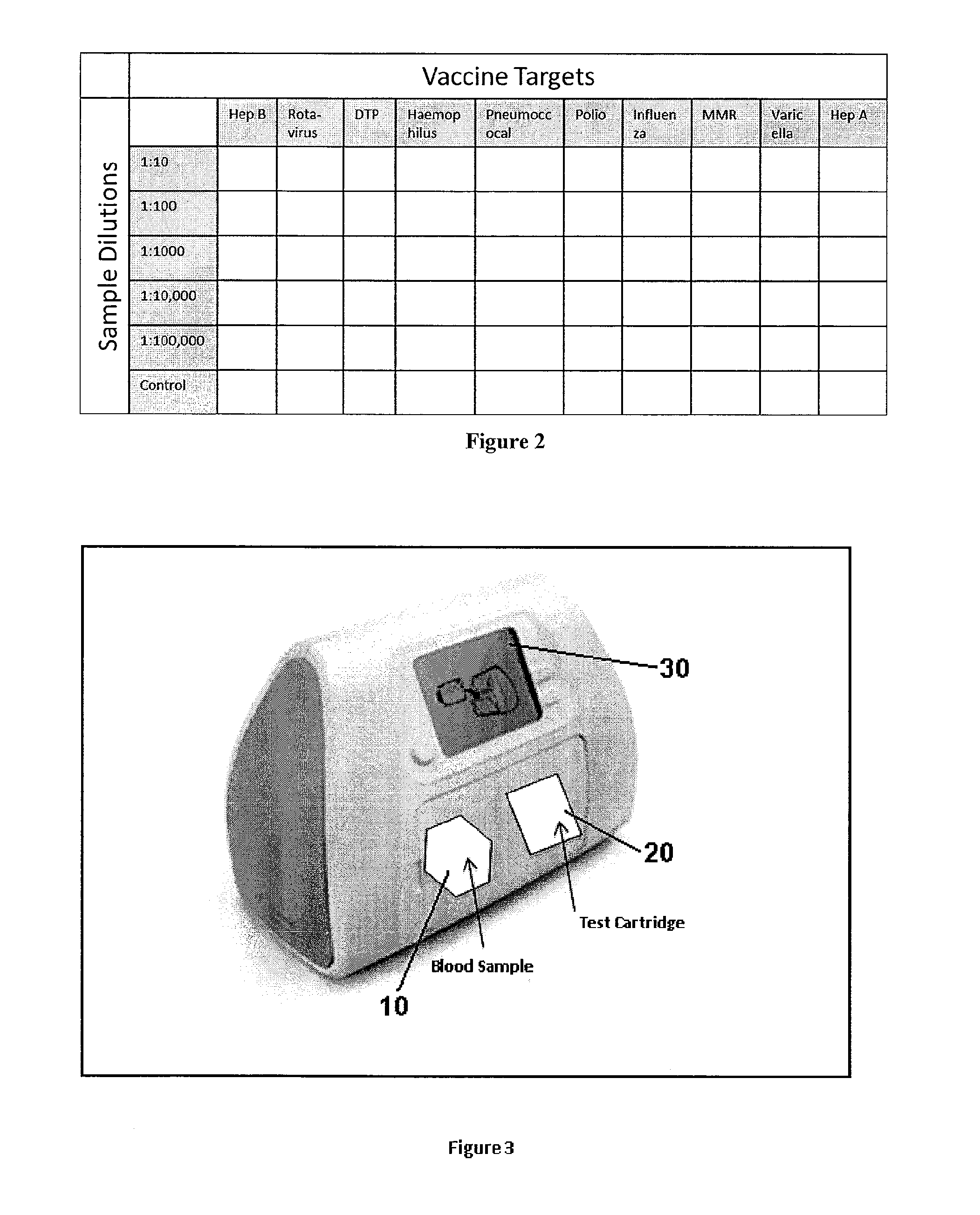
[0050]Referring now to the drawings in greater detail, FIG. 1 shows a detailed view of an exemplary sample dilution profile. The sample from the patient will be diluted per the protocols of the test procedure, typically in series fashion. Using fluidic devices (such as microfluidics or robotic pipetting) the sample can be mixed with dilution buffer solution to create the first dilution (e.g. 1:10) which is one part sample and nine parts buffer. The same or alternative dilutions can be conducted on dilutions of a given dilution step. In the Figure we illustrate a simple dilution system, but the point of care device can make virtually any dilution pool by taking the appropriate amount of sample combined with the specified amount of buffer. The figure is only an illustration of one such dilution profile.
[0051]The biological sample can be a fluid (e.g., blood, sera, lymph fluid, urine, tears, saliva or the like), a tissue (such as marrow, hair follicles, or the like). In the case of non...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com