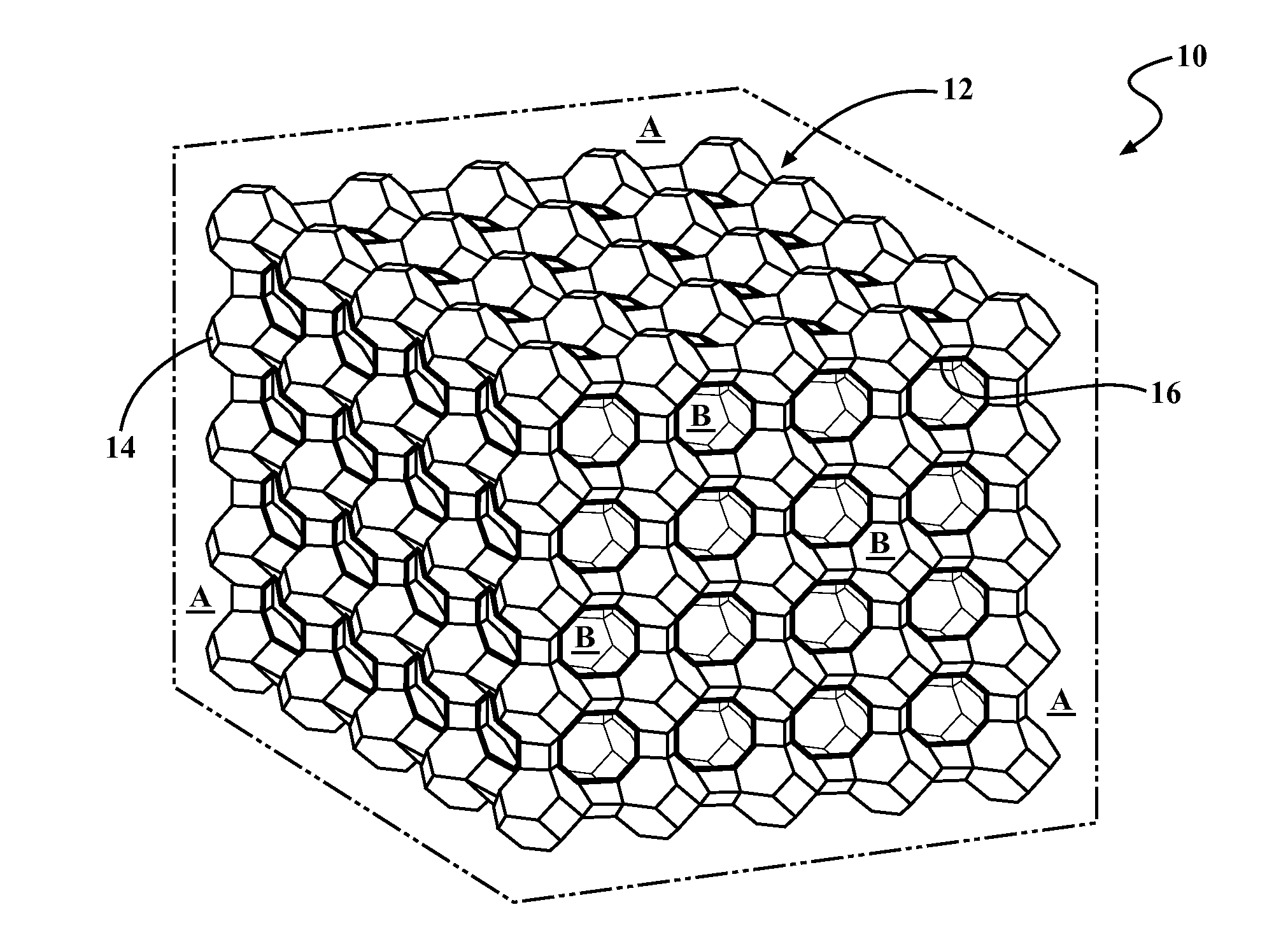
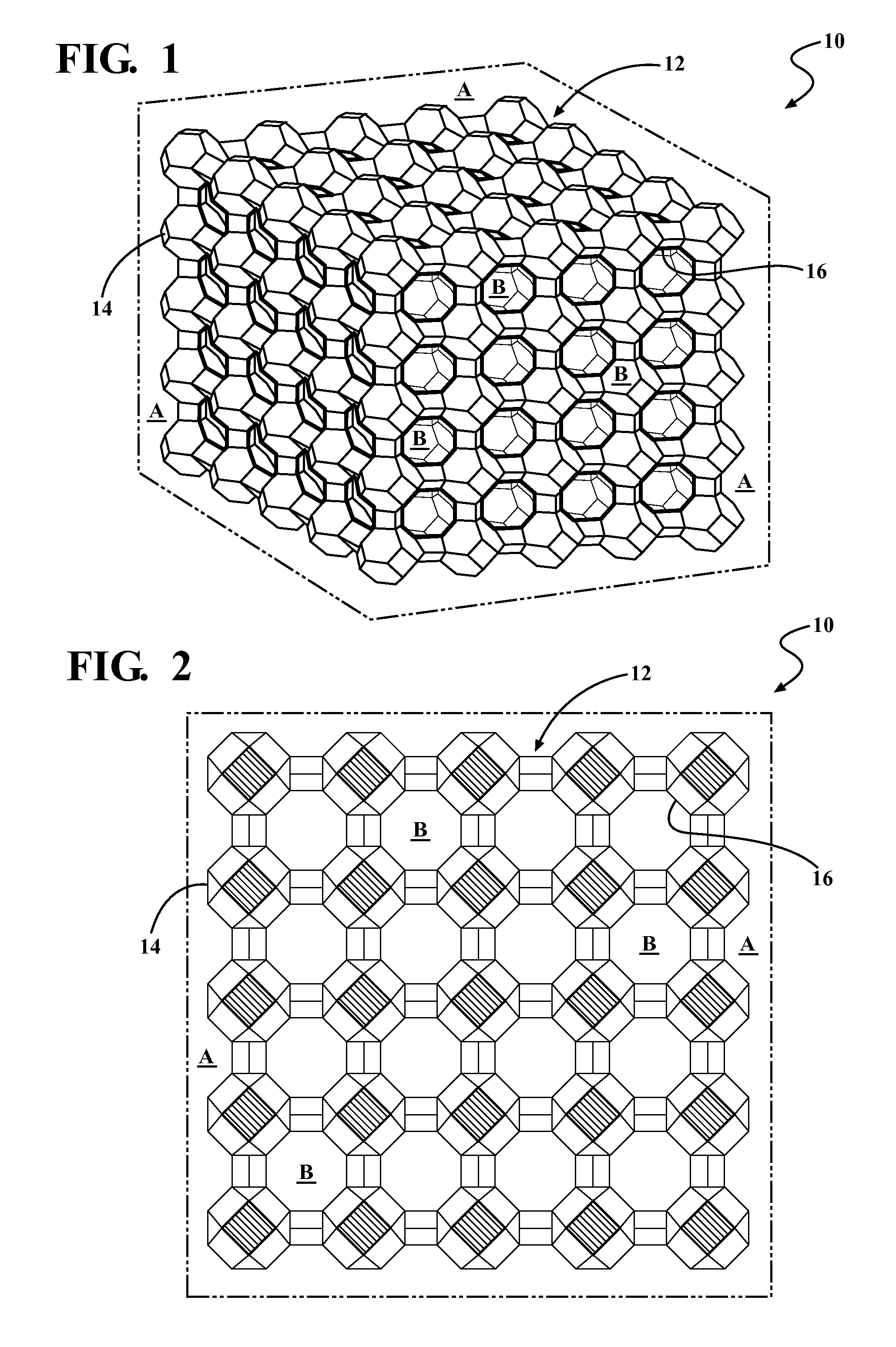
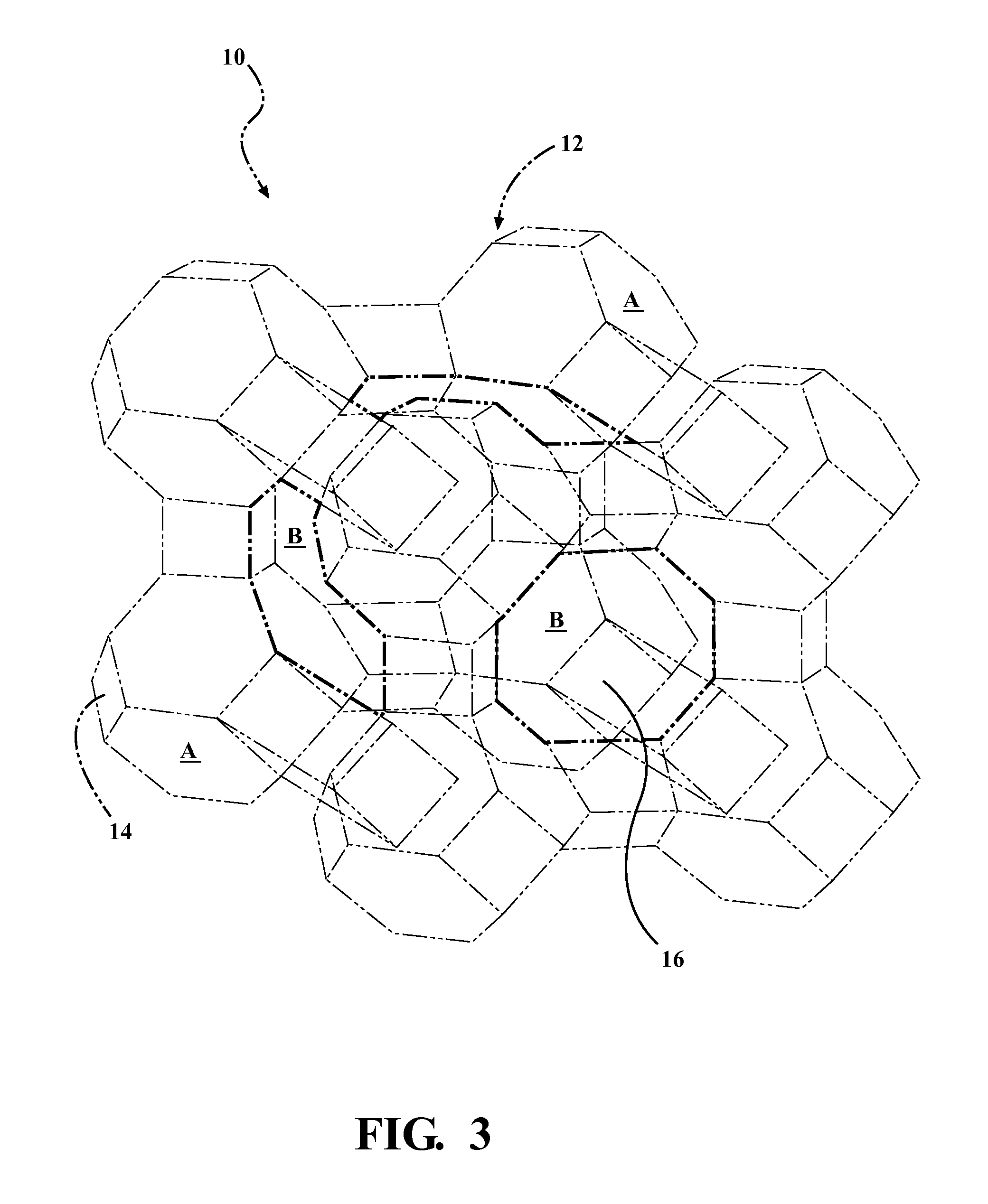
Catalyst For Decomposing A Plastic
a technology of catalysts and plastics, applied in the direction of physical/chemical process catalysts, organic compounds/hydrides/coordination complexes catalysts, metal/metal-oxide/metal-hydroxide catalysts, etc., can solve the problems of plastics that are not recycled and re-used, potential environmental pollution risks, and neither method is particularly efficient, so as to reduce energy consumption and reduce potential environmental pollution , the effect of reducing dependen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
##ventive example 1
Inventive Example 1
[0053]A 0.4% solution of PdCl2 is prepared by the dilution of 0.4 g of PdCl2 in 100 g of solvent wherein the solvent includes water acidified with HCl such that the solvent is visibly clear. 50 ml of the 0.4% solution of PdCl2 is combined with a 13X molecular sieve which includes an exterior surface and at least one pore. The 13X molecular sieve is present in an amount such that total pore volume of the 13X molecular sieve is more than 50 ml. This typically ensures that the 50 ml 0.4% solution of PdCl2 impregnates the at least one pore through capillary action. The 13X molecular sieve combined with PdCl2 is then dried for 24 hours at a temperature of 110° C. 0.5 g of sodium borohydride is then combined with 60 ml of water to form a sodium borohydride solution. The sodium borohydride solution is then combined with the dried 13X molecular sieve to reduce the PdCl2 to Pd and then dispose the Pd in the at least one pore wherein the Pd is the reducing catalyst componen...
##ventive example 2
Inventive Example 2
[0055]A 0.4% solution of PdCl2 is prepared by the dilution of 0.4 g of PdCl2 in 100 g of solvent wherein the solvent includes water acidified with HCl such that the solvent is visibly clear. 50 ml of the 0.4% solution of PdCl2 is combined with a 13X molecular sieve which includes an exterior surface and at least one pore. The 13X molecular sieve is present in an amount such that total pore volume of the 13X molecular sieve is more than 50 ml. This typically ensures that the 50 ml 0.4% solution of PdCl2 impregnates the at least one pore through capillary action. The 13X molecular sieve combined with PdCl2 is then dried for 24 hours at a temperature of 110° C. 0.5 g of sodium borohydride is then combined with 60 ml of water to form a sodium borohydride solution. The sodium borohydride solution is then combined with the dried 13X molecular sieve to reduce the PdCl2 to Pd and then dispose the Pd in the at least one pore wherein the Pd is the reducing catalyst componen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com