Influenza vaccines
a technology of influenza vaccine and antigen, applied in the field of medicine, can solve the problems of not being suited to controlling pandemics, constant antigenic changes in ha, emergence of variant or new virus strains, etc., and achieve the effects of increasing the cross-reactive immune response, reducing the potency of influenza ha-containing antigen, and reducing the risk of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
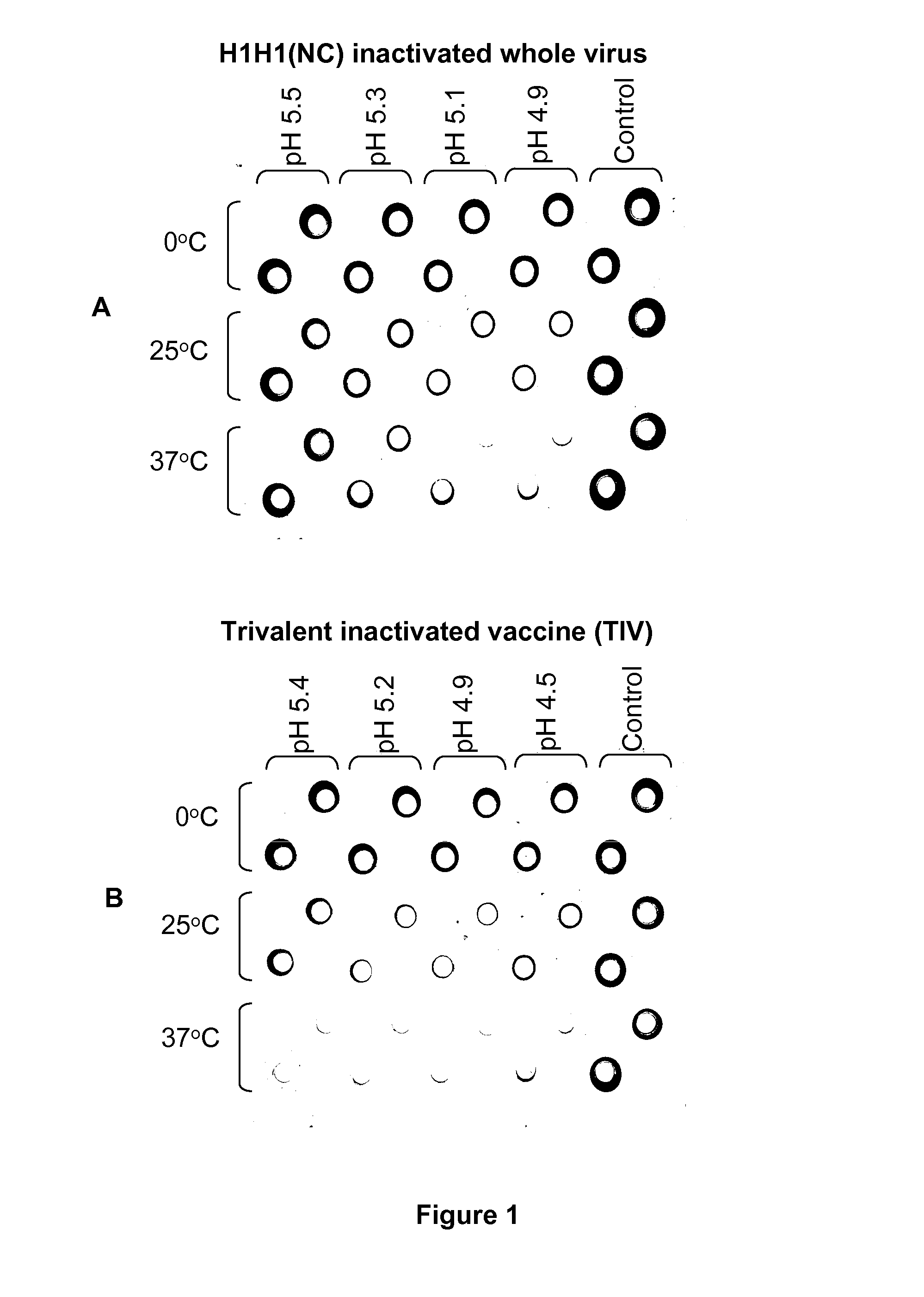
Potency of Inactivated Influenza Whole Virus (WV) Antigens Following Low pH Treatment
[0066]Inactivated WV antigens from three virus strains (A / New Caledonia / 20 / 99, H1 H1 (H1N1 NC); A / Wisconsin / 67 / 2005, H3N2 (H3N2 Wis); B / Malaysia / 2506 / 2004 (B Mal) were used. They were produced by purification from infected MDCK cells and inactivation with formaldehyde (1 / 4000 dilution or 0.01%) for at least three days at 4° C. The inactivation was confirmed by titration in chicken eggs and plaque assay in MDCK cells.
[0067]For low pH treatment, antigens in phosphate buffered saline (PBS, pH 7.2; ˜2.0 mg / ml) were diluted 1:10 with 20 mM sodium citrate buffers containing 150 mM NaCl (pH 4.6-5.4 in 0.2 increments) to bring the pH of antigens to 4.9-5.5 with a final antigen concentration at ˜0.2 mg / ml. The antigens were then kept at 0° C. (on ice), 4° C., 25° C. (room temperature), or 37° C. for 15 min before the pH was adjusted with an appropriate volume of 1 M Tris HCl buffer (pH 8.0) back to the origi...
example 2
Potency of Trivalent Inactivated Vaccine (TIV) Following Low pH Treatment
[0071]A TIV (Fluzone, 2006-2007) which contains the antigens from the same three virus strains described in Example 1 was also evaluated for potency after low pH treatment under different conditions. The vaccine was similarly treated, but with appropriate volumes of 0.1 M HCl added to the vaccine to adjust the pH to a similar range before neutralization with an equal volume of 0.1 M NaOH. The potency of H1N1 NC strain was measured. The results were similar to those obtained with inactivated WV antigens as described above (FIG. 1B and Table 3). However, TIV was more susceptible to low pH treatment than the WV antigen in that at any given condition, potency loss was greater with TIV. This may be related to the fact that TIV is made of split antigens. A complete potency loss (100%) was obtained at 37° C. for all pH levels tested (FIG. 1B and Table 3). Similarly, hemagglutination activity only decreased by 2 fold o...
example 3
Induction of Increased Cross-Reactive Antibody Responses by Low pH-Treated Antigens
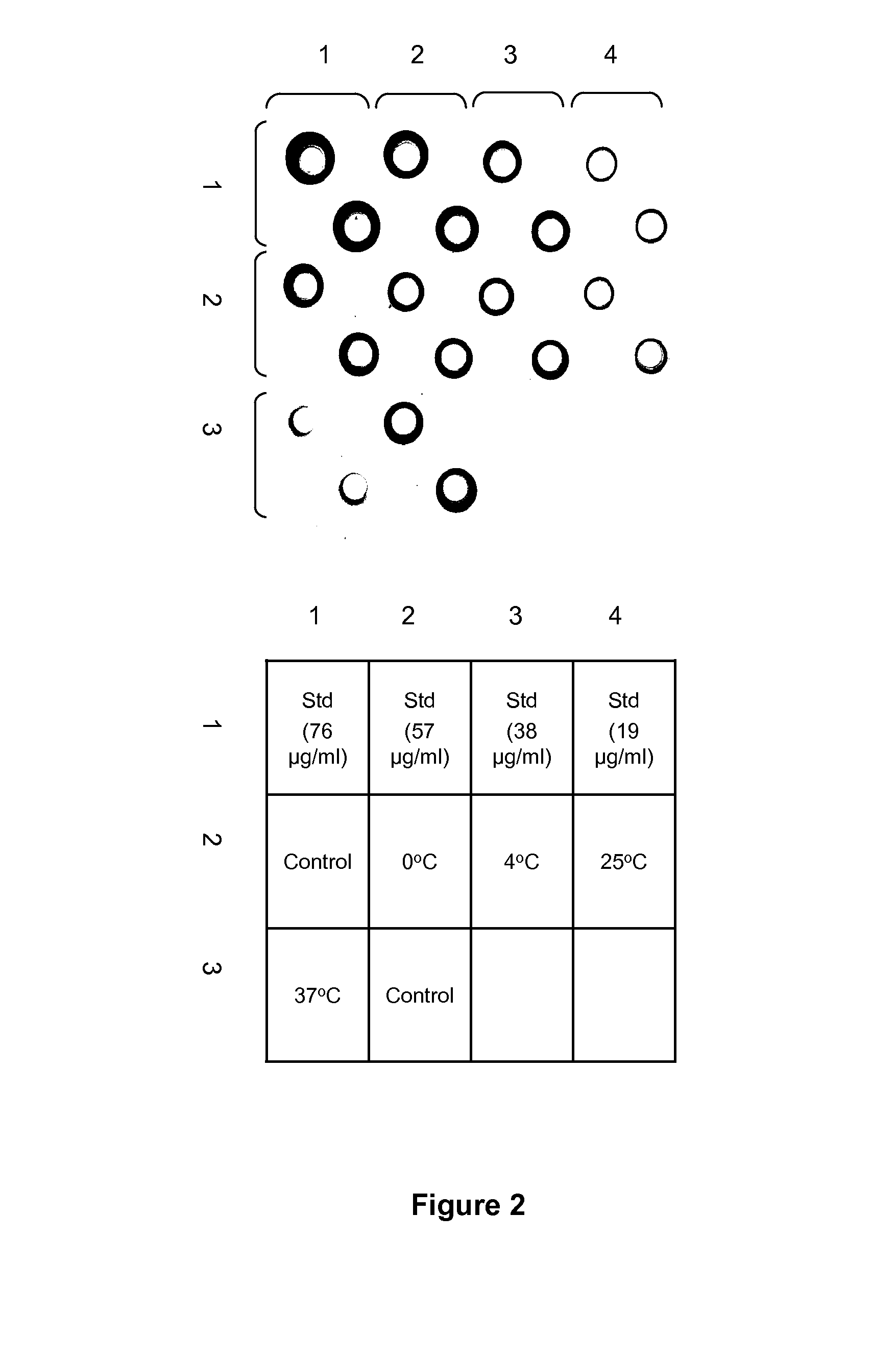
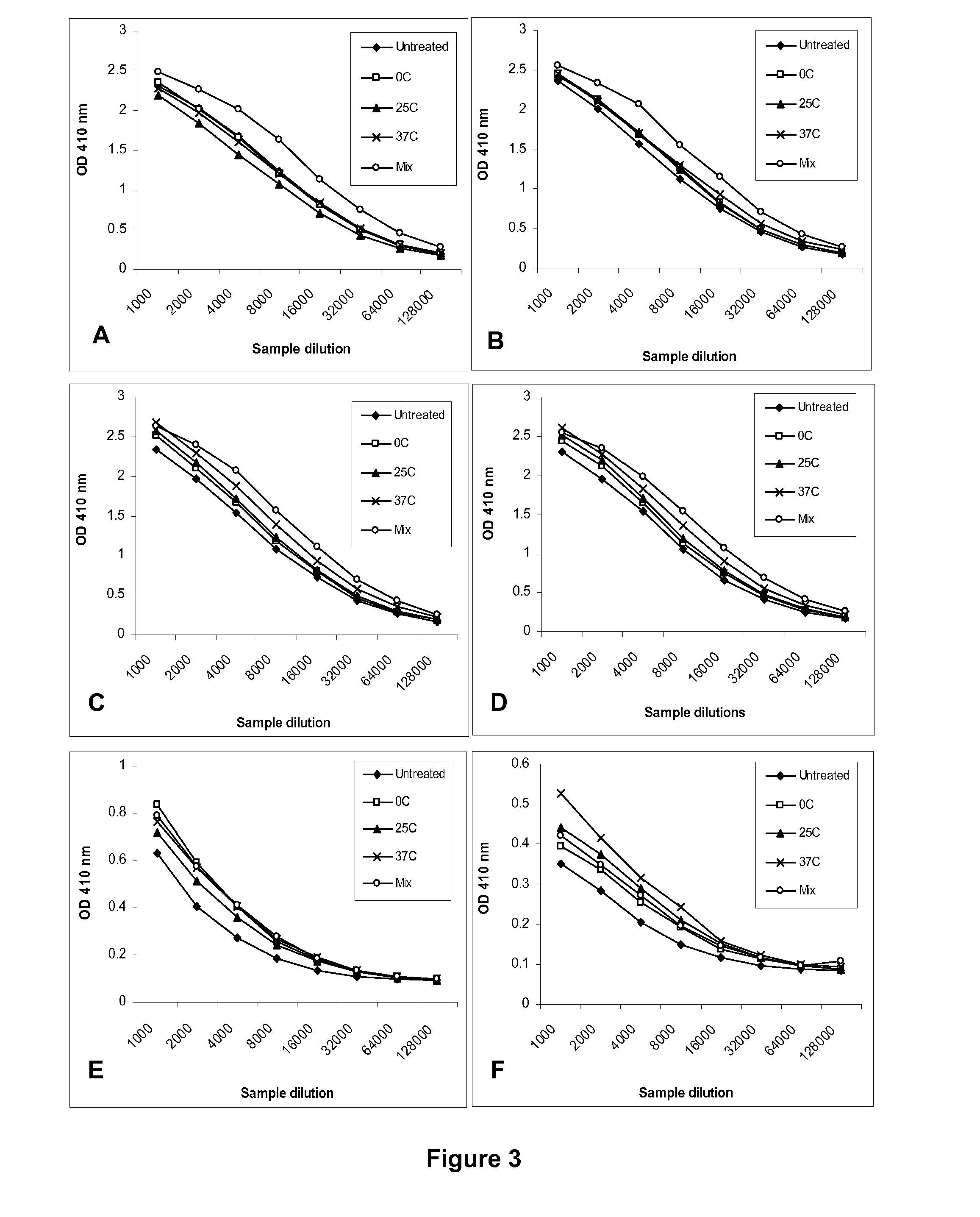
[0073]Low pH-treated H1N1 NC WV antigens with different levels of potency loss were used to immunize 6-8 weeks old female Balb / c mice (n=7) together with the untreated control. The antigens were treated at pH 5.1 and different temperatures (0, 25, and 37° C.) and the potency (HA, μg / ml) was determined as described in Example 1. The treated antigens exhibited the potency reduction in correlation with the treatment conditions, but retained the original hemagglutination activity (Table 5).
[0074]Mice were immunized at the same antigen dose based on total proteins which was equivalent to 1 μg HA / mouse for untreated antigen by intramuscular injection twice, 4 weeks apart. A group receiving a mixture of antigens containing equal parts of the untreated antigen and the one treated at 37° C. was also included (Table 5). The mixed antigen exhibited a similar level of potency as the untreated antigen as expected ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com