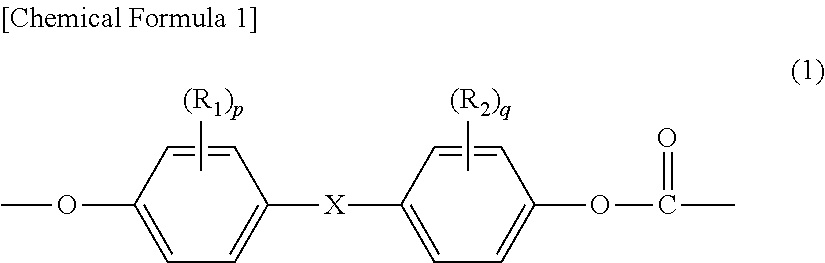
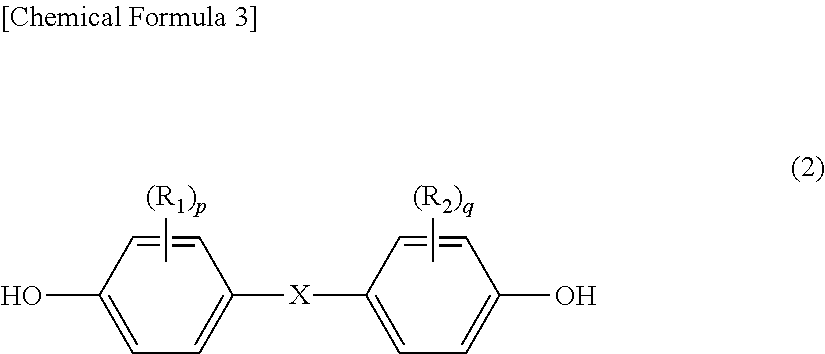
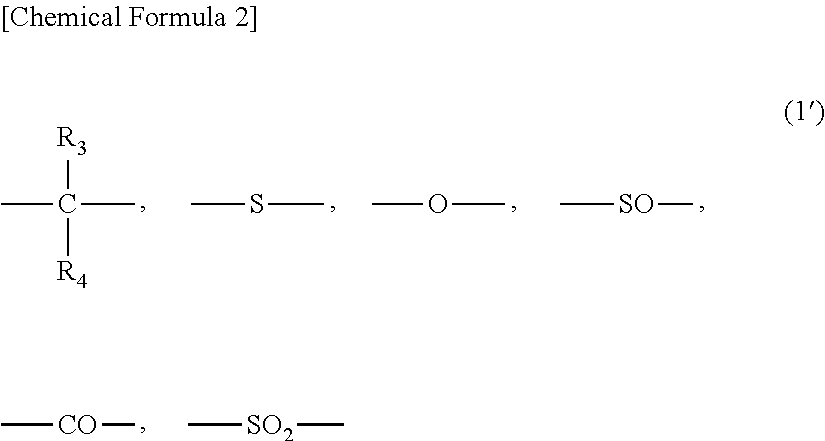Method for producing branched aromatic polycarbonate resin
a technology of aromatic polycarbonate and resin, which is applied in the field of method for producing branched aromatic polycarbonate resin, can solve the problems of poisonous phosgene using the producing method, difficult molding of large-sized products, and dripping of resin by self-weight, and achieves the desired branching degree easily, good quality inherently, and sufficient molecular weight-increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0238]In 300 cc of a four-necked flask equipped with a stirrer and a distillation apparatus were charged 45.5 g (0.20 mol) of BPA, 48.0 g (0.22 mol) of DPC and 0.5 μmol / mol (which means “0.5 μmol based on 1 mol of BPA”) of cesium carbonate as a catalyst, and the mixture was heated to 180° C. under nitrogen atmosphere and stirred for 5 minutes.
[0239]Thereafter, the pressure was adjusted to 27 kPa (200 torr) and simultaneously the temperature of the mixture was raised to 205° C. for 35 minutes. Thereafter, the mixture was maintained at 27 kPa (200 torr) and at 205° C. for 15 minutes to carry out transesterification reaction. Further, the temperature of the mixture was raised to 215° C. for 10 minutes, and the pressure was adjusted to 24 kPa (180 torr). At 215° C., the pressure was maintained to 24 kPa (180 torr) for 10 minutes, subsequently, the temperature of the mixture was raised to 235° C. for 10 minutes, and the pressure was adjusted to 20 kPa (150 torr). Moreover, the temperatur...
example 2
[0241]In 300 cc of a four-necked flask equipped with a stirrer and a distillation apparatus were charged 45.5 g (0.20 mol) of BPA, 48.1 g (0.22 mol) of DPC, 0.05 g (0.000373 mol) of TMP and 0.5 μmol / mol (which means “0.5 μmol based on 1 mol of BPA”) of cesium carbonate as a catalyst, and the mixture was heated to 180° C. under nitrogen atmosphere and stirred for 5 minutes.
[0242]Thereafter, the pressure was adjusted to 27 kPa (200 torr) and simultaneously the temperature of the mixture was raised to 205° C. for 35 minutes. Thereafter, the mixture was maintained at 27 kPa (200 torr) and at 205° C. for 15 minutes to carry out transesterification reaction. Further, the temperature of the mixture was raised to 215° C. for 10 minutes, and the pressure was adjusted to 24 kPa (180 torr). At 215° C., the pressure was maintained to 24 kPa (180 torr) for 10 minutes, subsequently, the temperature of the mixture was raised to 235° C. for 10 minutes, and the pressure was adjusted to 20 kPa (150 t...
example 3
[0244]In 300 cc of a four-necked flask equipped with a stirrer and a distillation apparatus were charged 45.5 g (0.20 mol) of BPA, 48.0 g (0.22 mol) of DPC and 1.0 μmol / mol (which means “1.0 μmol based on 1 mol of BPA”) of sodium hydrogen carbonate as a catalyst, and the mixture was heated to 180° C. under nitrogen atmosphere and stirred for 5 minutes.
[0245]Thereafter, the pressure was adjusted to 27 kPa (200 torr) and simultaneously the temperature of the mixture was raised to 205° C. for 35 minutes. Thereafter, the mixture was maintained at 27 kPa (200 torr) and at 205° C. for 15 minutes to carry out transesterification reaction. Further, the temperature of the mixture was raised to 215° C. for 10 minutes, and the pressure reduction degree was adjusted to 24 kPa (180 torr). At 215° C., the pressure was maintained to 24 kPa (180 torr) for 10 minutes, subsequently, the temperature of the mixture was raised to 235° C. for 10 minutes, and the pressure was adjusted to 20 kPa (150 torr)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| structural viscosity index | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com