System and method for optogenetic therapy
a technology of optogenetic therapy and system and method, applied in the field of system and method of optogenetic therapy, can solve the problems of significant clinical downsides and inhibition of such signals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
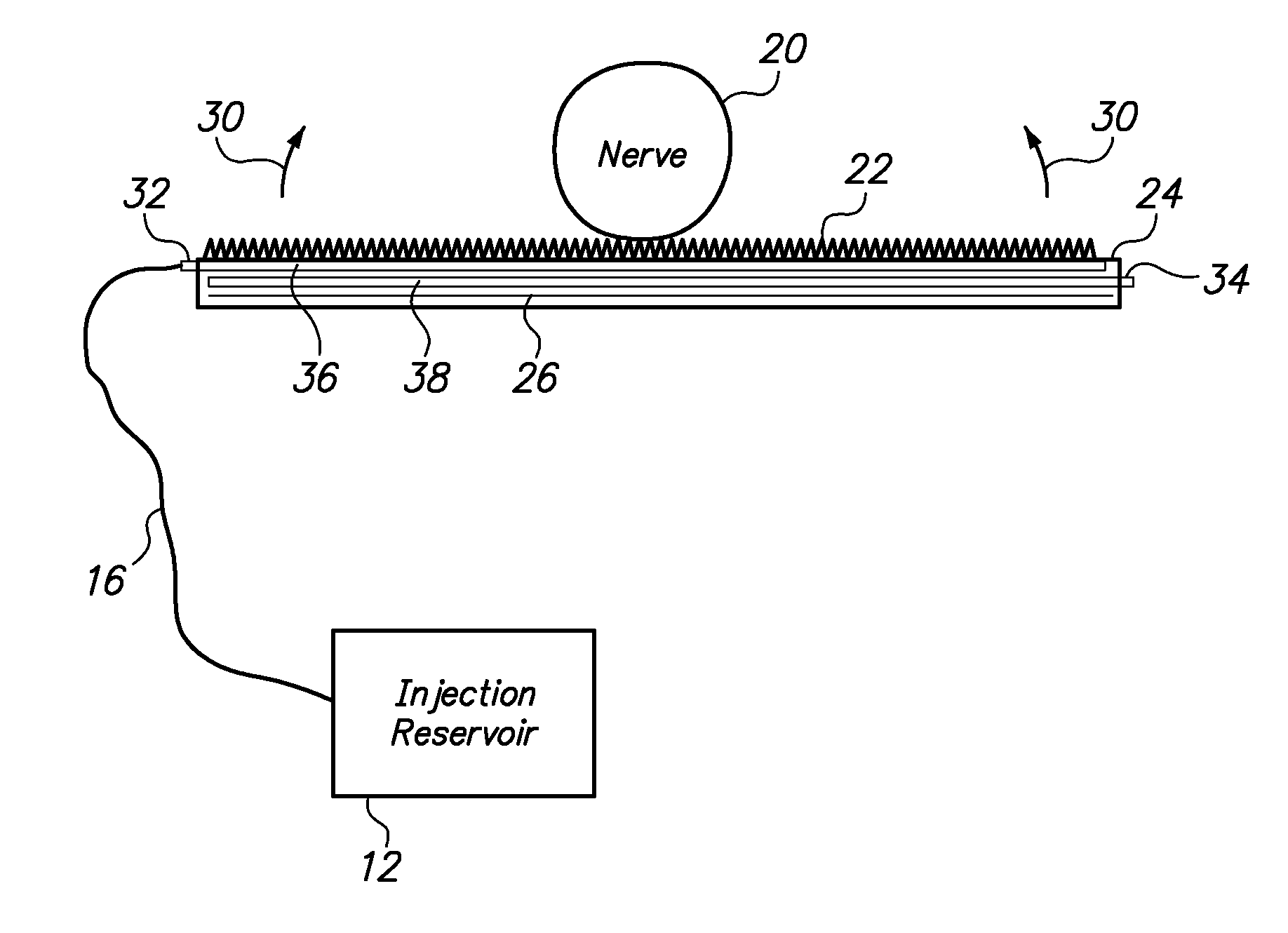
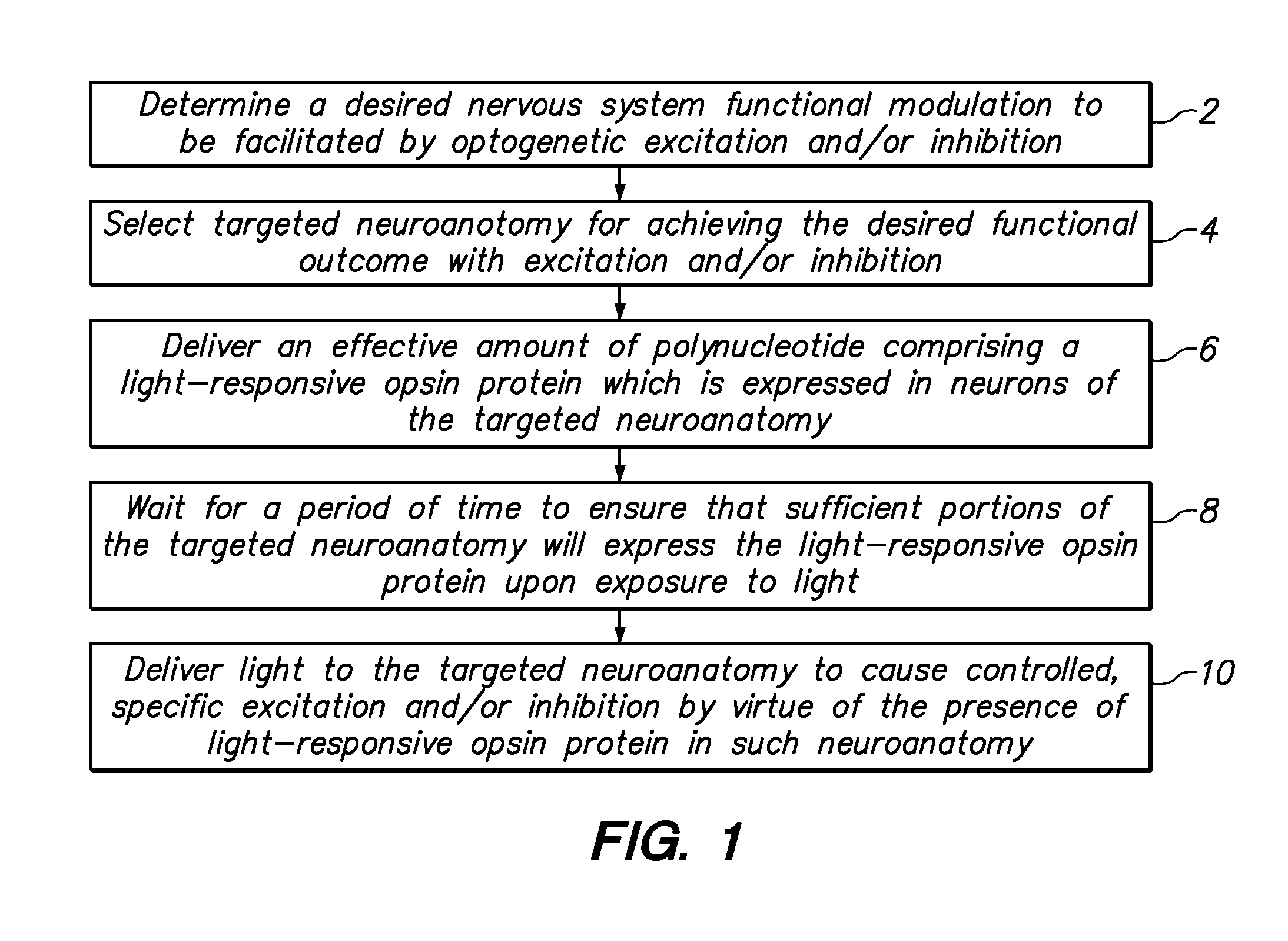
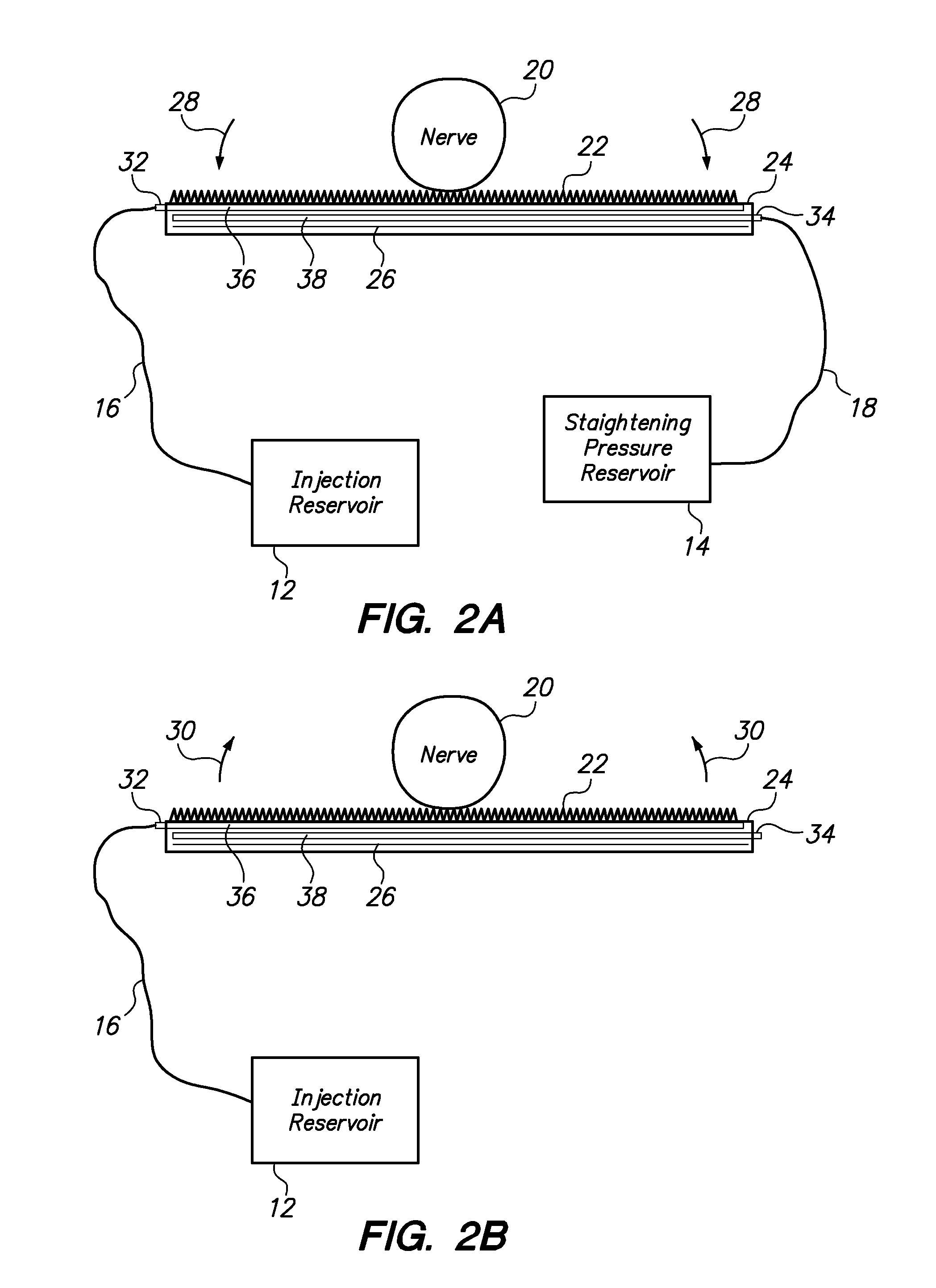
[0042]Referring to FIG. 1, from a high-level perspective, an optogenetics-based neuromodulation intervention involves determination of a desired nervous system functional modulation which can be facilitated by optogenetic excitation and / or inhibition (2), followed by a selection of neuroanatomic resource within the patient to provide such outcome (4), delivery of an effective amount of polynucleotide comprising a light-responsive opsin protein which is expressed in neurons of the targeted neuroanatomy (6), waiting for a period of time to ensure that sufficient portions of the targeted neuroanatomy will indeed express the light responsive opsin protein upon exposure to light (8), and delivering light to the targeted neuroanatomy to cause controlled, specific excitation and / or inhibition of such neuroanatomy by virtue of the presence of the light-responsive opsin protein therein (10).
[0043]While the development and use of transgenic animals has been utilized to address some of the afo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| yellow-wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com