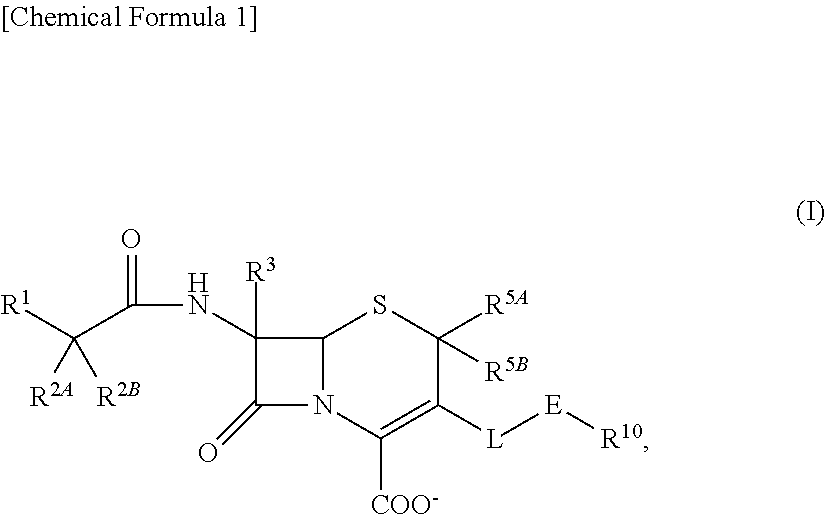
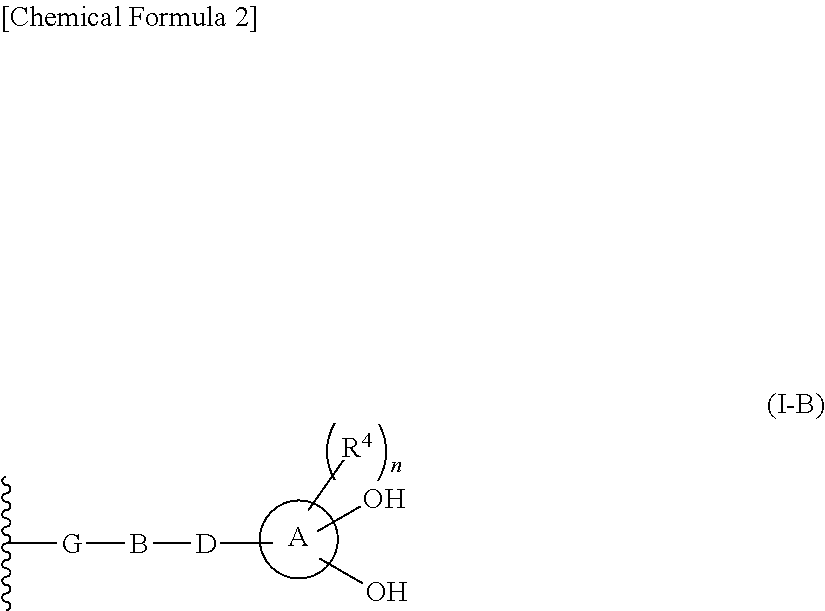
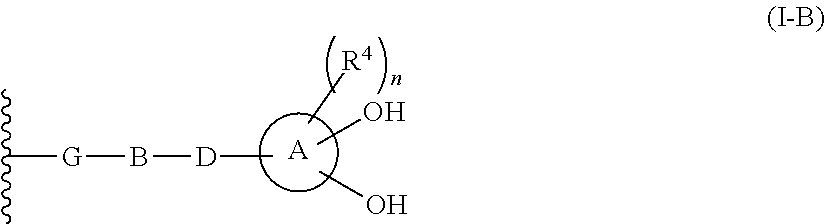
2-substituted cephem compounds
a cephem compound and cephem technology, applied in the field of cephem compounds with substituents, can solve the problems of not disclosing a compound having a substituent, clinical problems, etc., and achieve the effects of high bioavailability, high blood concentration, and excellent kinetics in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
Synthesis of Compound X-1
[0301]
Step (1): Compound X-1a→Compound X-1b
[0302]Compound X-1a (26.47 g, 51.2 mmol) which was synthesized according to the synthesis in U.S. Pat. No. 4,463,172A1 was dissolved into dioxane (200 mL), and thereto was then added 4 mol / L hydrochloric acid solution in dioxane (25.6 ml, 102 mmol) at rt. The mixture was stirred at rt for 1 hour. The reaction mixture was concentrated under reduced pressure. The precipitated solid was then collected by filtration, and washed with diisopropyl ether / dichloromethane to yield compound X-1b (21.1 g, 75%).
[0303]1H-NMR (CDCl3) δ: 7.37-7.26 (11H, m), 7.03-6.99 (2H, m), 6.87 (1H, s), 6.36 (1H, d, J=8.7 Hz), 5.63-5.59 (1H, m), 5.23-5.20 (2H, m), 4.31 (1H, d, J=12.3 Hz), 4.09 (1H, d, J=12.3 Hz), 3.86 (2H, s), 1.99 (3H, s).
Step (2): Compound X-1b→Compound X-1c
[0304]Compound X-1b (5.53 g, 10 mmol) was dissolved into dichloromethane (60 mL), and thereto was then added dropwise a solution of m-chloroperoxybenzoic acid (3.45 g, 13 m...
reference example 2
Synthesis of Compound X-2
[0315]
Step (1): Compound X-1e+Compound X-2a→Compound X-2b
[0316]Compound X-1e (8.3 g, 15 mmol) and compound X-2a (10.4 g, 18 mmol) were used to synthesize the target compound in the same way as in step 4 and 5 of Reference Example 1.
[0317]Yielded amount: 10.4 g, (70%)
[0318]1H-NMR (CDCl3) δ: 8.27 (1H, d, J=8.1 Hz), 8.09 (1H, s), 7.43-7.29 (11H, m), 7.23 (2H, d, J=8.5 Hz), 6.94 (1H, s), 6.82 (2H, d, J=8.5 Hz), 5.92 (1H, dd, J=8.1, 4.9 Hz), 5.36 (1H, dd, J=8.4, 4.6 Hz), 5.23 (1H, d, J=4.9 Hz), 5.14 (1H, d, J=11.9 Hz), 5.05 (1H, d, J=11.9 Hz), 4.85 (1H, d, J=12.3 Hz), 4.23 (1H, d, J=12.3 Hz), 4.00 (1H, q, J=7.4 Hz), 3.76 (3H, s), 2.92 (1H, dd, J=16.4, 8.4 Hz), 2.83 (1H, dd, J=16.4, 4.6 Hz), 1.57 (3H, d, J=7.4 Hz), 1.54 (9H, s), 1.41 (9H, s).
Step (2): Compound X-2b→Compound X-2
[0319]Compound X-2b (10.4 g, 11 mmol) was used to synthesize the target compound in the same way as in step 6 of Reference Example 1.
[0320]Yielded amount: 10.7 g, (95%) 1H-NMR (CDCl3) δ: 8.3...
reference example 3
Synthesis of Compound X-3 and X-24
[0321]
Step (1): Compound X-3a→Compound X-3b→Compound X-3c
[0322]To a pre-cooled solution of compound X-3a (50 g, 97 mmol), which was synthesized according to the synthesis in Tetrahedron Letter, 37, 1971-1974 (1996), in dichloromethane (450 mL) at −10° C. was added peracetic acid (19.82 g, 102 mmol, 37% Wt). The mixture was stirred at −10 to −5° C. To the resulting mixture was added a solution of sodium bisulfite (12.1 g, 116 mmol) in water (200 mL). Water (150 mL) was added to the mixture, and then an organic layer was separated. The organic layer was washed with water (250 mL), 10% aqueous solution of sodium chloride (250 mL). The aqueous layers were successively extracted with dichloromethane (150 mL). The combined organic layers were dried over magnesium sulfate and filtered. To the filtrated was added dimethylformamide (200 mL) and then the solution was concentrated. The residue was placed in a reaction bottle with dimethylformamide (30 mL) and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Antimicrobial properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com