Nanoparticle drug conjugates
a technology of nanoparticles and conjugates, applied in the field of nanoparticle conjugates, can solve the problems of poor interstitial permeation of liposomes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental examples
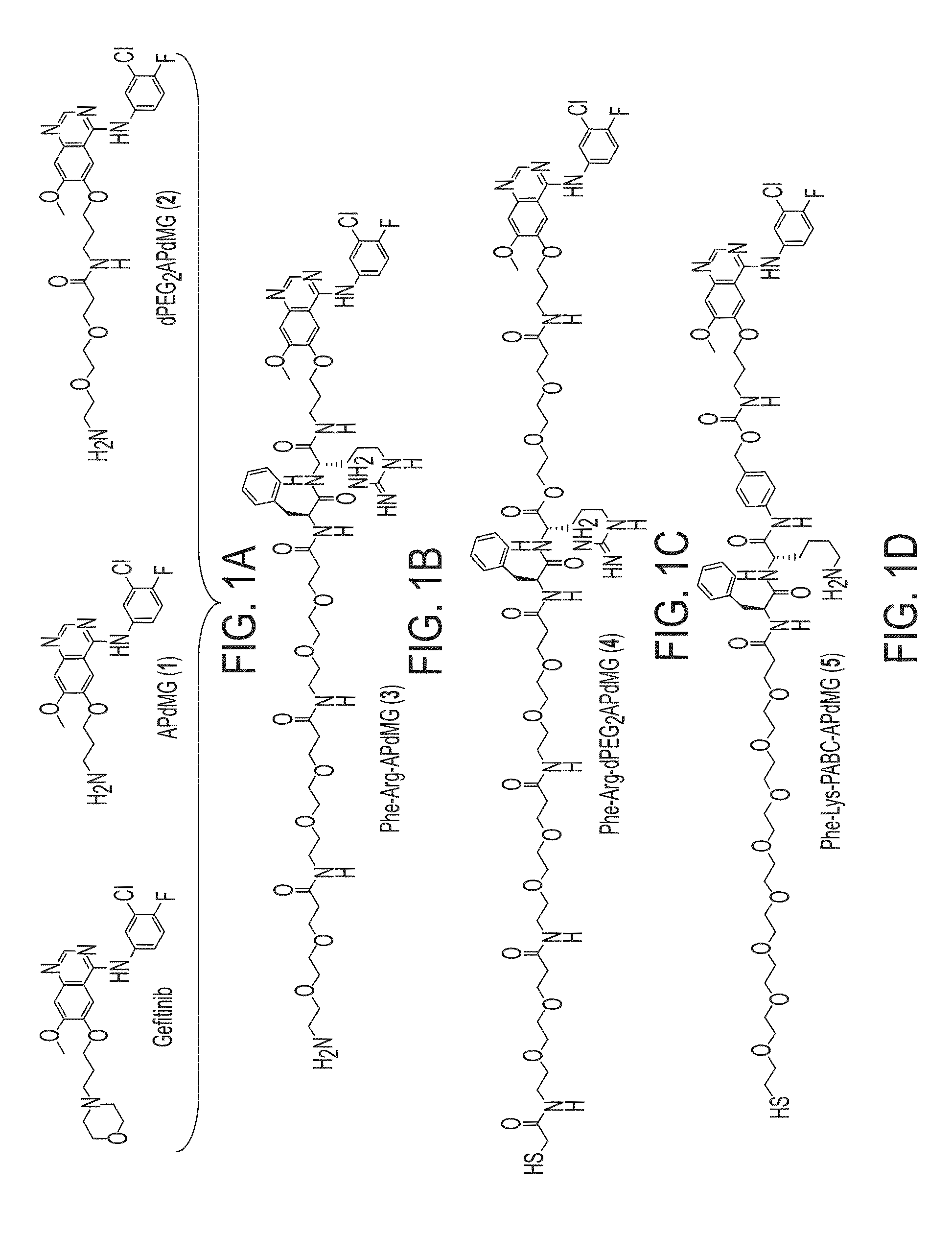
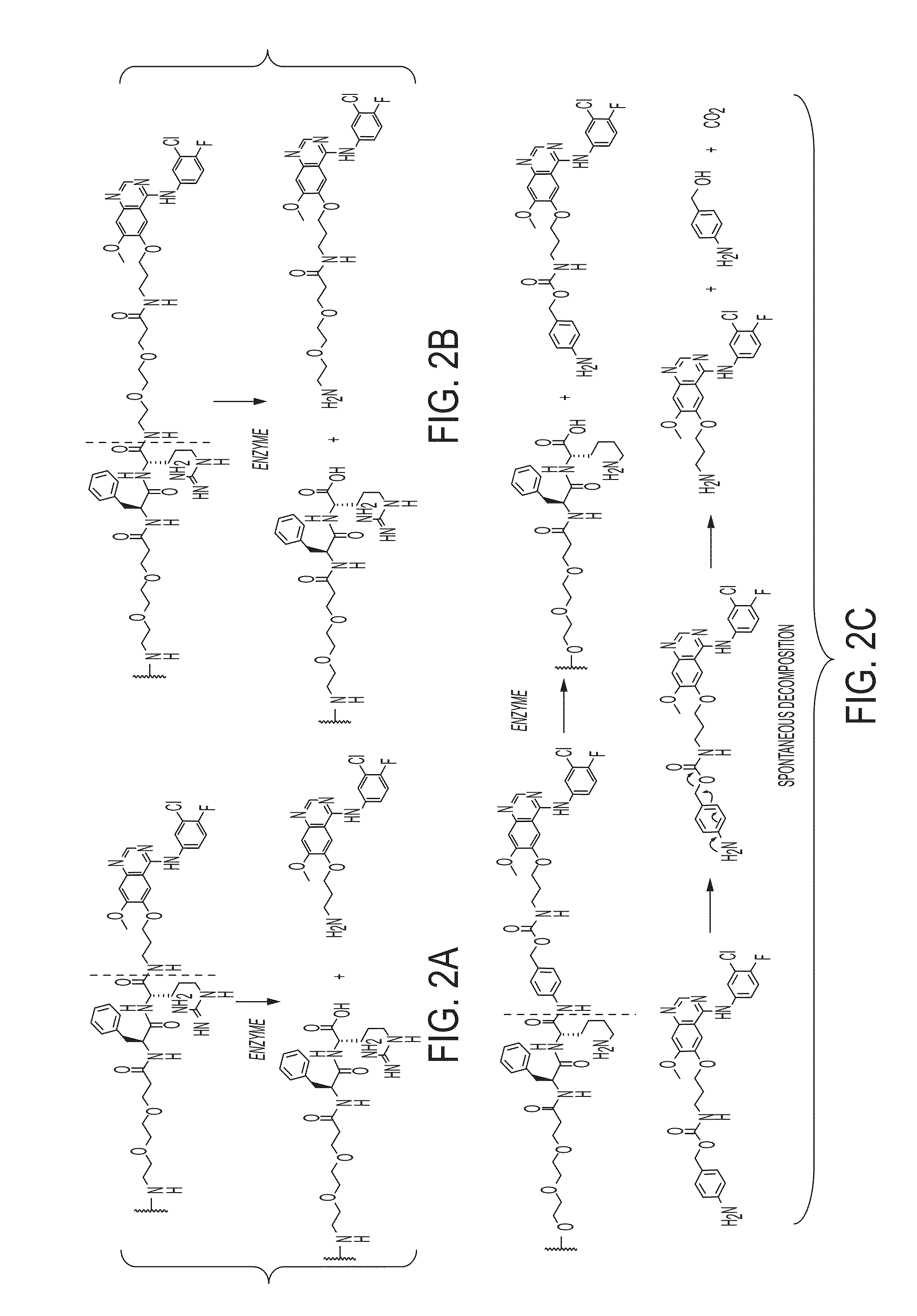
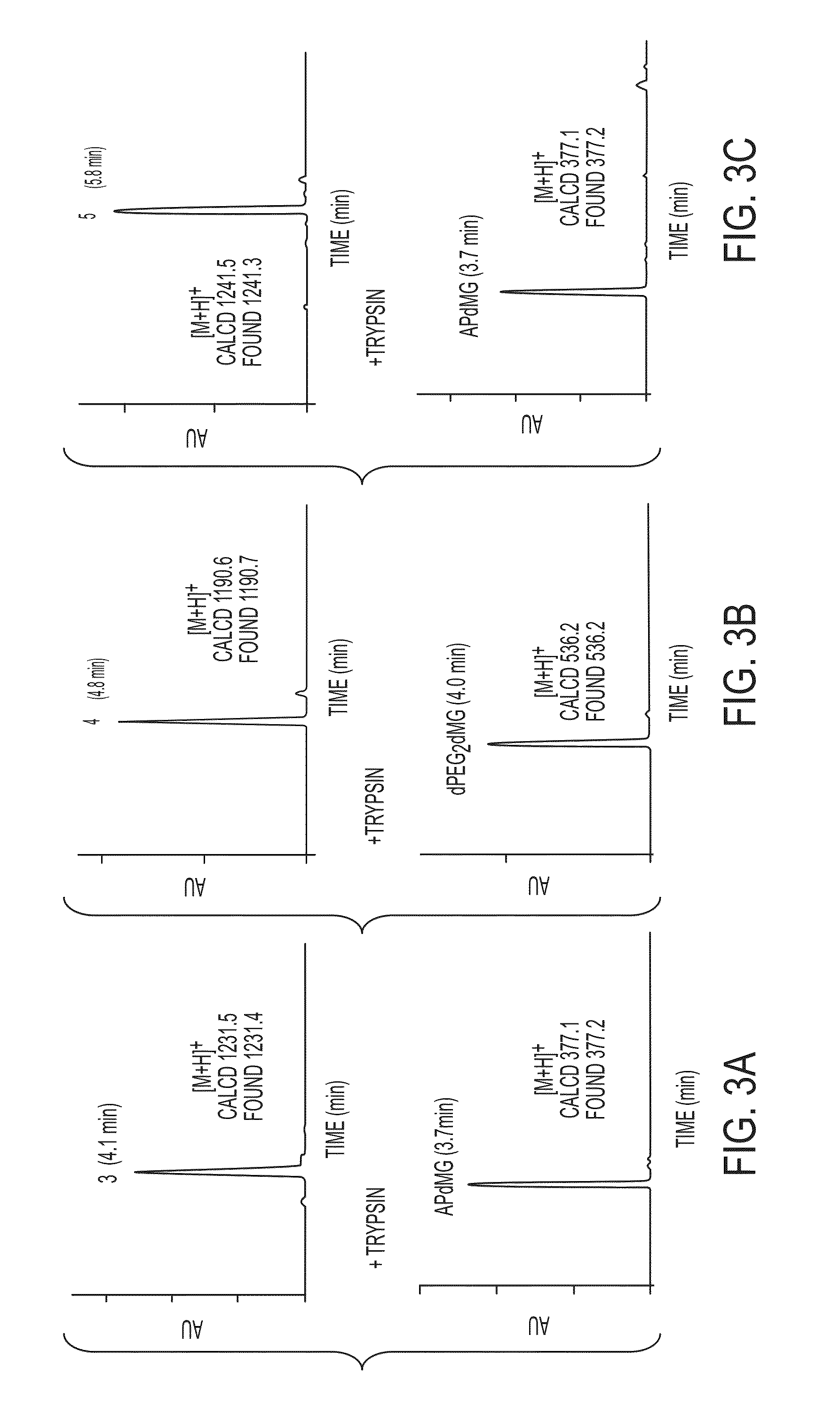
[0086]One example demonstrates exemplary synthesis of nanoparticle drug conjugates (e.g., silica-based nanoparticle platform with covalently attached drug molecules) and their characterization and preliminary biological evaluations.
[0087]With the commercial availability of des-morpholino-gefitinib (dMG), the desired aminopropyl-dMG (APdMG) was obtained through a nucleophilic substitution (e.g., in one step) of Boc protected amino propyl bromide, followed by acid deprotection (FIG. 1A, FIG. 10 (Scheme 1)). Additionally, the gefitinib analogue 2, which is described in further detail below, was readily obtained from 1 by coupling Fmoc-dPEG2-COOH, with a subsequent base deprotection step (FIG. 1A, FIG. 11 (Scheme 2)). To ensure that APdMG 1 and dPEG2APdMG 2 have retained activity against EGFR, H1650 cells were treated with the compounds and analyzed by western blot to assess phospho-Tyr168 levels in EGFR. The H1650 cells are a model human tumor-derived non-small-cell lung cancer (NSCLC)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com