Methods and compositions to treat cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Screening Test Compounds
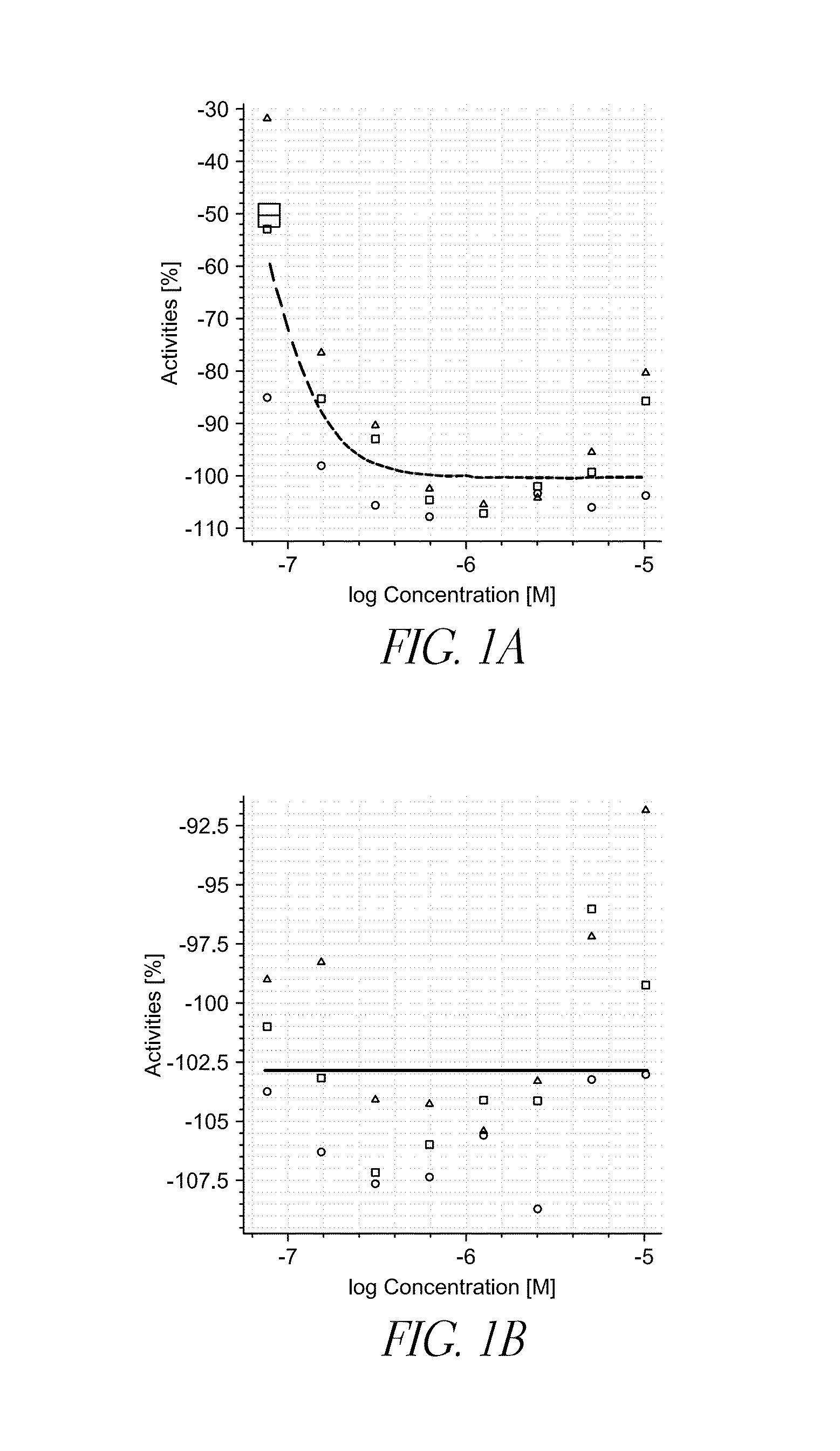
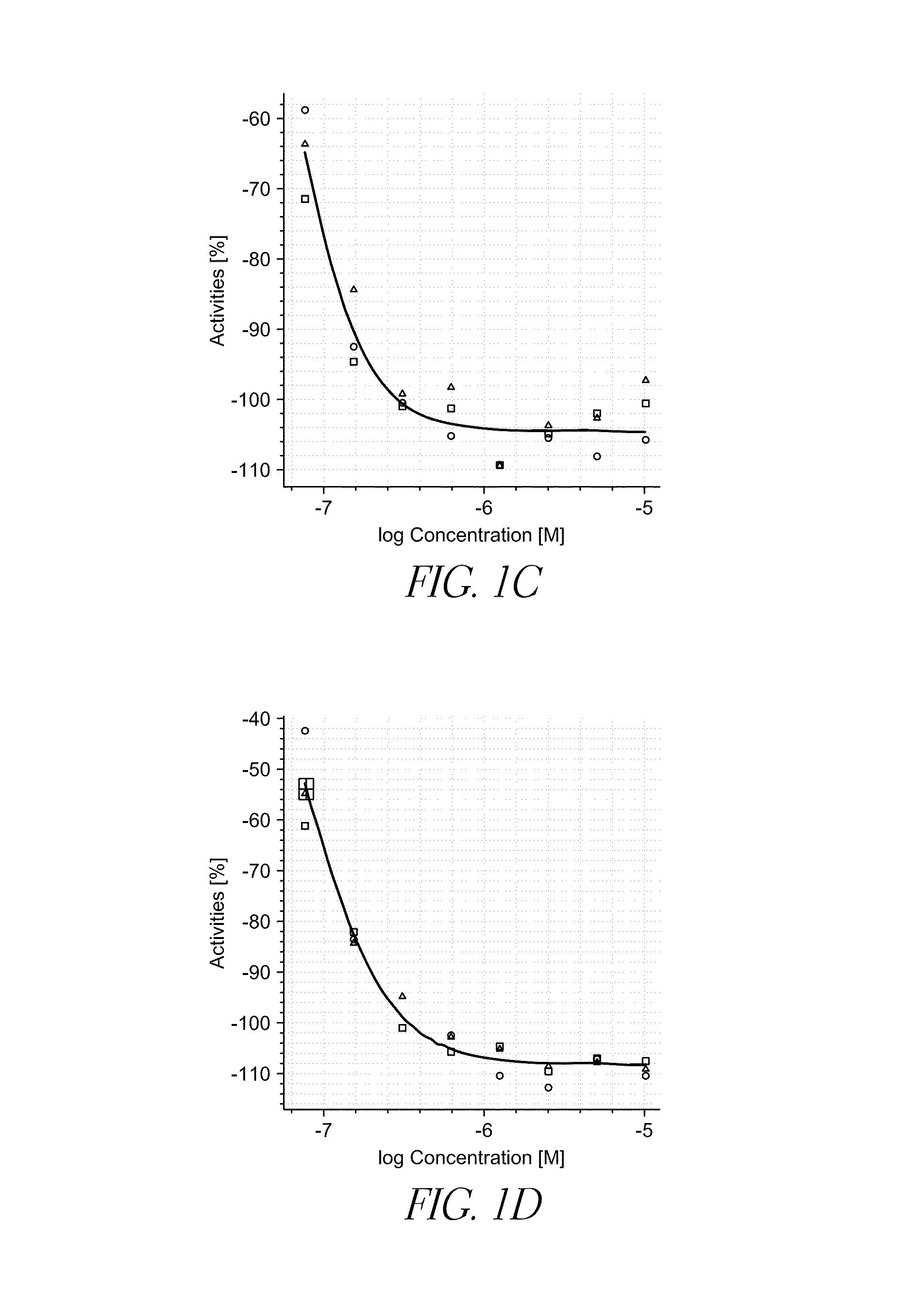
[0091]A library of compounds was screened for activity to reduce expression of midkine in vitro. HEK293 cells were stably transfected with the pGL4 vector comprising a midkine promoter coupled to a luciferase reporter gene. Midkine expression was upregulated by treating the cells with retinoic acid, and then treating the cells with a test compound. Changes in luciferase production were measured to access the inhibitory activity of the test compounds. The primary screen included 44,000 test compounds. Test compounds that reduced cell viability by more than 50% were eliminated from the screen. From the primary screen, 351 test compounds were further examined. From the secondary screen, 78 test compounds were examined using a dose-response assay, and the EC50 for the test compounds was determined. Example test compounds are listed in TABLE 1.
TABLE 1LowerUpperExample95%95%compoundEC50confidenceconfidenceNo.Structure(nM)limit (nM)limit (nM)114.04010.73018.3702312....
example 2
In Vitro and in Vivo Analysis of iMDK
Materials and Methods
[0093]Reagents: 3-[2-(4-fluorobenzyl)imidazo[2,1-beta][1,3 ]thiazol-6-yl]-2H-chromen-2-one (iMDK) purchased from ChemDiv (San Diego, Calif.) was dissolved in DMSO.
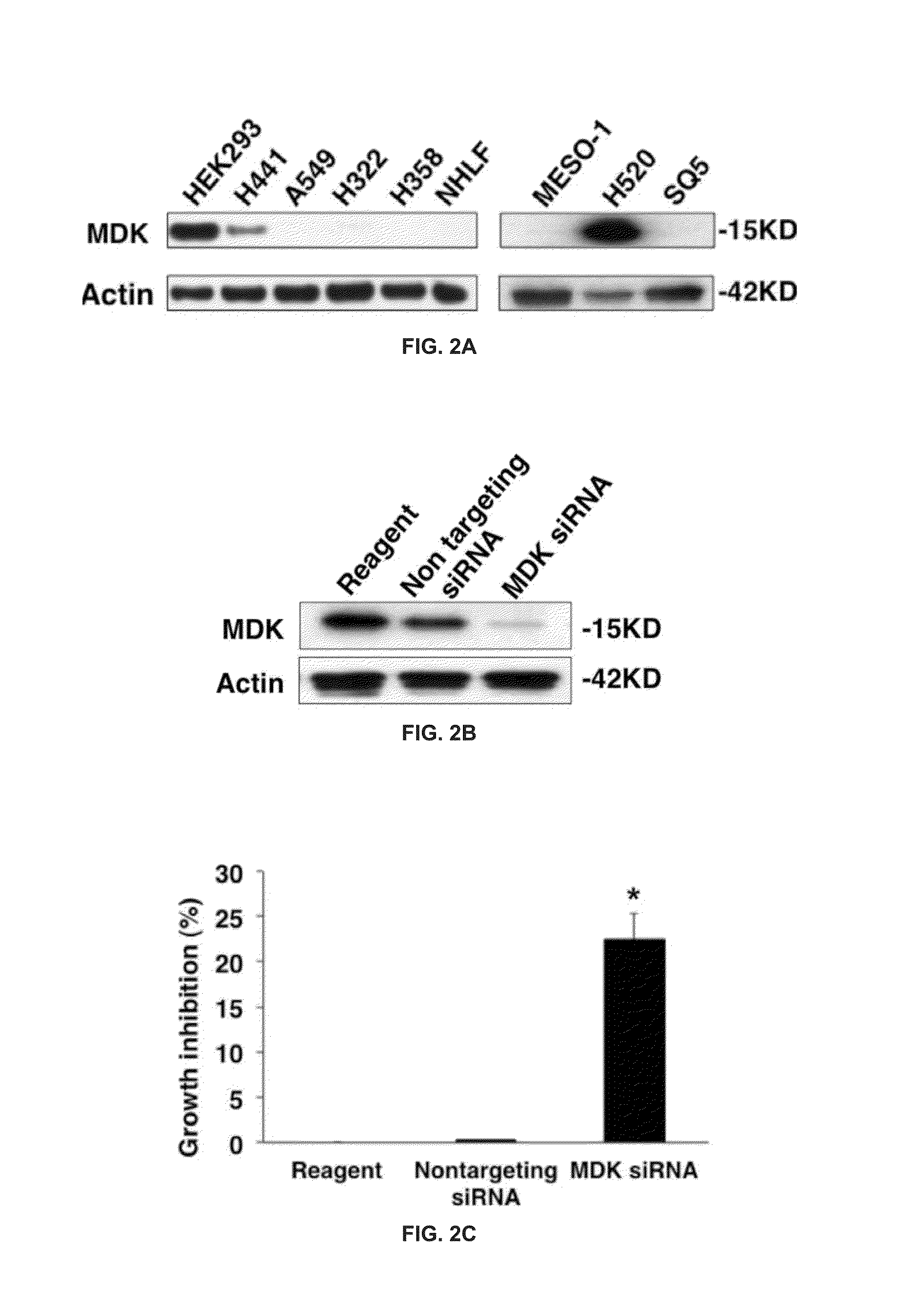
[0094]Cell Lines and Culture Conditions: the human pulmonary adenocarcinoma cells H322, H358, H441 and A549 and the human lung squamous cell carcinoma cells H520 were obtained from the American Type Culture Collection (Manassas, Va.) and grown in RPMI 1640 (H322, H358, H520) or high glucose Dulbecco's modified Eagle medium (H441, A549 cells) supplemented with 10% heat-inactivated fetal bovine serum. The human malignant mesothelioma cells ACC-MESO-1 (MESO-1) obtained from JCRB Cell Bank (Osaka, Japan) and the human lung squamous cell carcinoma cells SQ5 were grown in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum. The normal human lung fibroblasts (NHLF) obtained from Clonetics (San Diego, Calif.) were grown in high glucose Dulbecco's modified Ea...
example 3
Inhibition of Midkine Expression
[0113]Midkine protein expression in response to various concentrations of a compound from TABLE 2 in H441 was measured using Western blots. Briefly, H441 cells were treated with the compounds at the indicated final concentrations. 24 hours after treatment, cells were lysed and cell extracts were used for western blotting as described previously (Maeda Y, et al., J Biol Chem.; 281:9600-6). The western blot membranes were then incubated with anti-Midkine (cat #ab52637, abcam) and anti-Actin (cat #A2066, Sigma-Aldrich) and probed with horseradish peroxidase-coupled secondary antibodies. Western blots were developed by Western HRP Substrates (Millipore) according to the manufacturers' instructions. Western blots are shown in FIG. 8A and FIG. 8B. All compounds of TABLE 2 inhibited midkine expression.
TABLE 2Concentration to inhibitExampleCompound structuremidkine protein expression12100 nM 510 μM6 1 μM7100 nM 8 1 μM9 1 μM1010 μM
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com