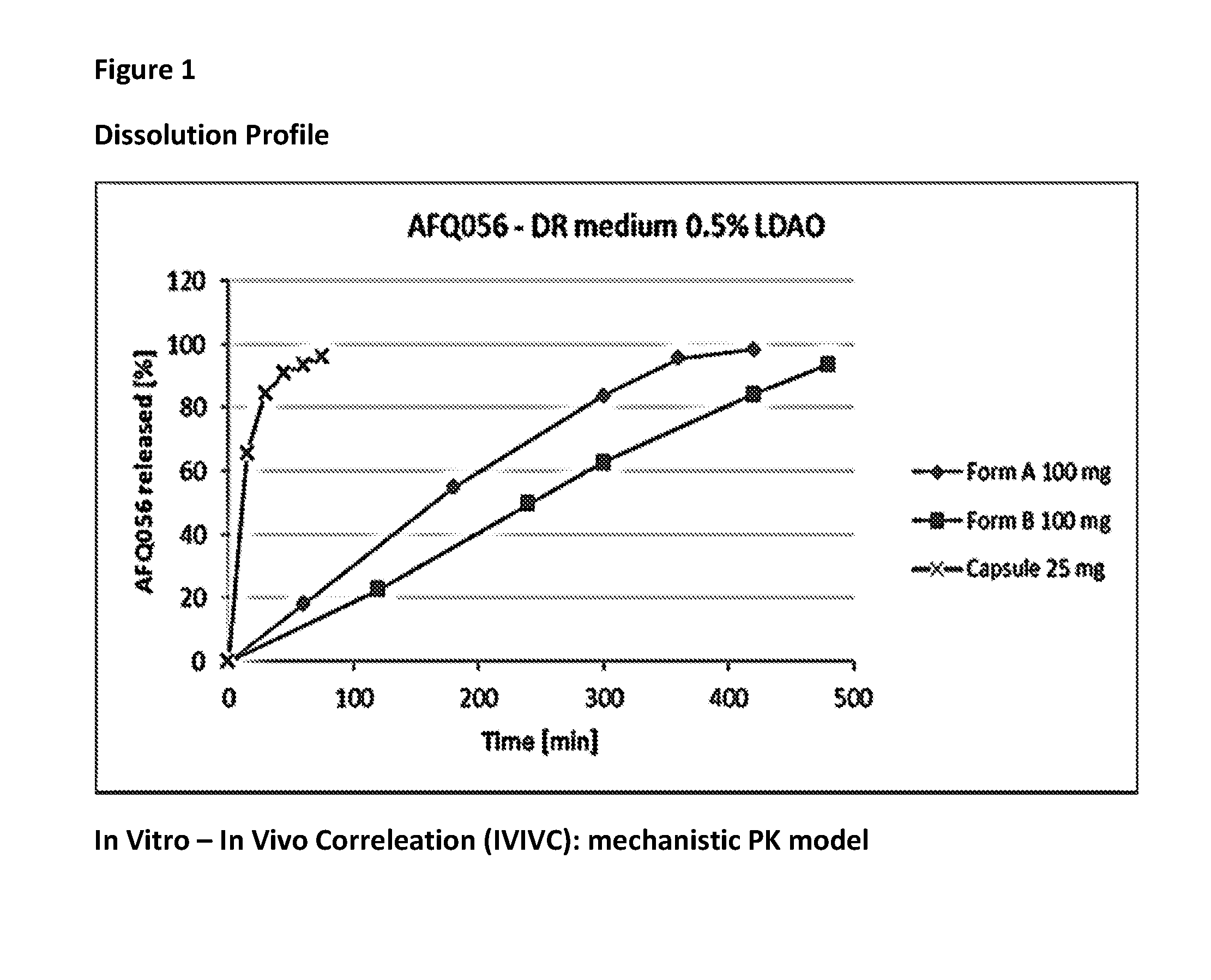
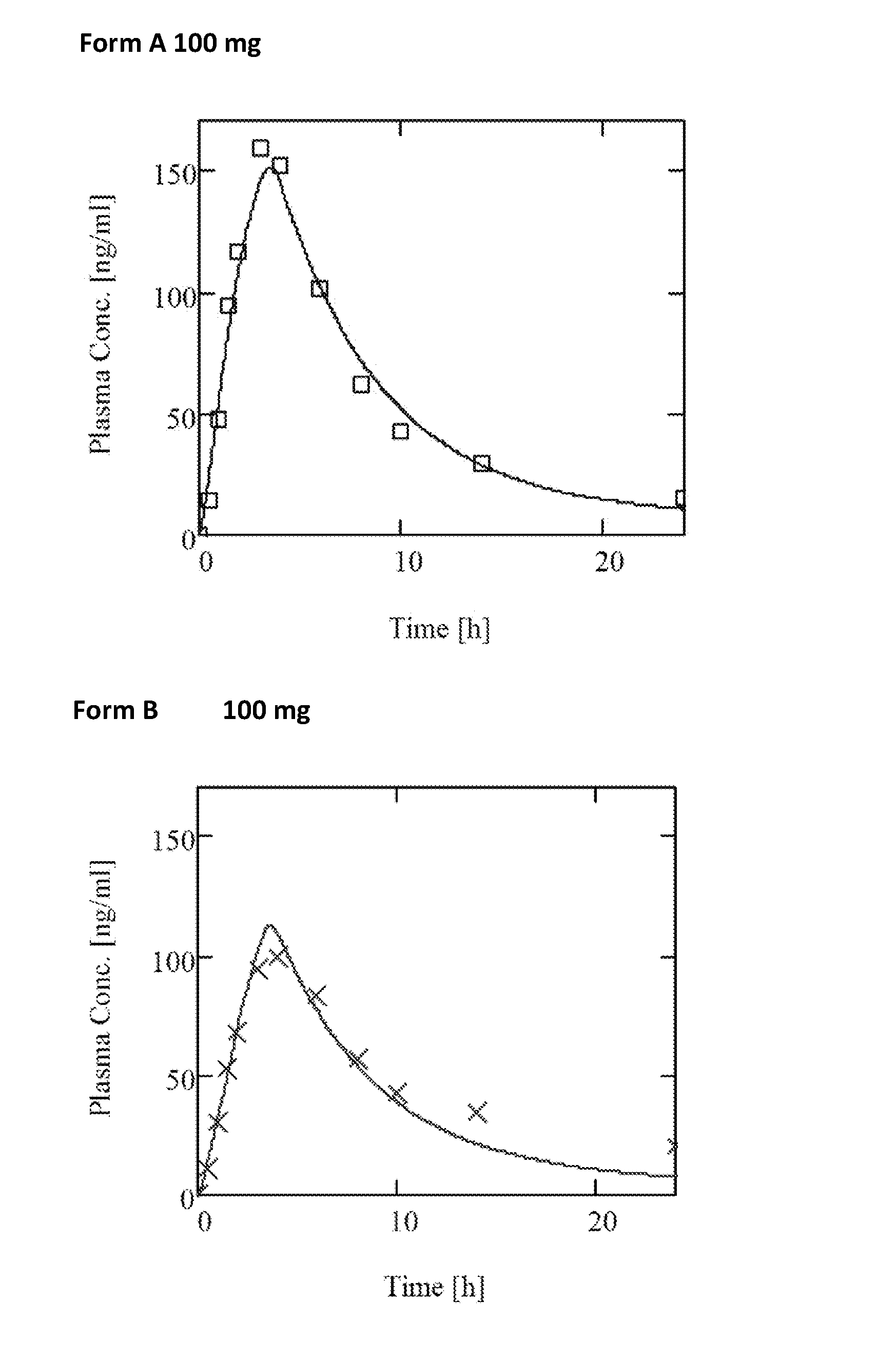
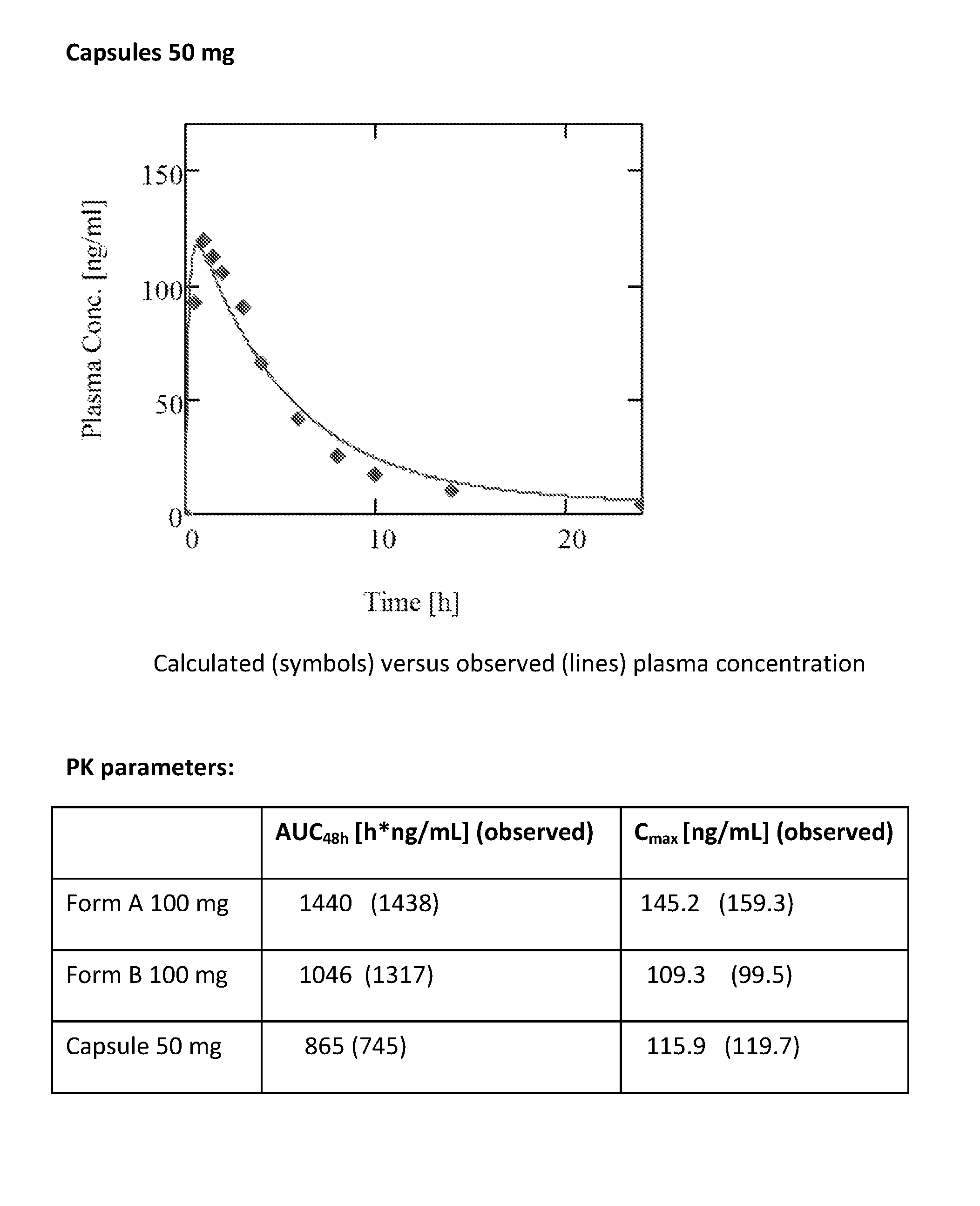
Modified release formulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0075]The following formulations (tablet core compositions) of AFQ056 are made:
TABLE 1IR-CapsForm AForm BForm C[mg / dose][mg / dose][mg / dose][mg / dose]AFQ056100.00100.00100.0099.00Lactose monohydrate100.0022.0022.0021.78Microcrystalline20.00———CelluloseSodium Starch16.2512.5012.5012.375glycolateHypromellose10.0042.8069.5071.775Colloidal Silicon1.250.901.000.99DioxideMagnesium stearate2.501.802.001.98Total250.00180.00207.00207.90IR-CapsForm AForm BForm C[%][%][%][%]AFQ05640.0055.5648.3147.62Hypromellose4.0023.7833.5734.52Lactose monohydrate40.0012.2210.6310.48Microcrystalline8.40———CelluloseSodium Starch6.506.946.045.95glycolateMagnesium stearate1.001.000.970.95Colloidal Silicon0.500.500.480.48Dioxide
[0076]Since previous oral formulations have exhibited an increased exposure upon concomitant intake of a high-fat meal, the extent of which has been found to be formulation-dependent, this study is designed to assess the food-effect (by administration of a high fat breakfast) on the PK of th...
example 2
[0090]The tablet core is formulated using common excipients for such pharmaceutical dosage forms. Release of the drug substance from the tablet core occurs through an erosion and diffusion mechanism, and is controlled by the hypromellose (type 2208) content of the formulated product. A pharmacokinetic study is performed using different 100 mg modified release tablet formulations in order to evaluate the impact of delaying release of the active ingredient.
[0091]The same ratio of excipients in the 100 mg tablet core is used to create the additional dosage strengths. The lower dosage strengths e.g. 25 mg, 50 mg and 75 mg use lactose monohydrate as compensation for drug substance in order to maintain the tablet weight and size. The tablet cores of dosage strengths less than or equal to 100 mg are compressed to round tablets possessing a diameter of 8 mm.
[0092]For the higher dosage strengths e.g. 150 mg, 200 mg and 250 mg, the tablet weight and size are increased. The same formulation pr...
example 3
[0094]Particle size distribution is an important factor in dissolution of the modified release forms of the present invention. The following experiments are performed to in order to determine how particle size effects dissolution at various time points.
[0095]FIG. 11 graphically depicts the percentage of AFQ056 dissolved after 45 minutes versus particle size at x90. As can be discerned from the figure, particle size distribution is a key factor in dissolution rate and thus the performance of the MR Form. The drug substance has a particle size distribution of x10≦50 μm, x50≦100 μm and x90≦200 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com