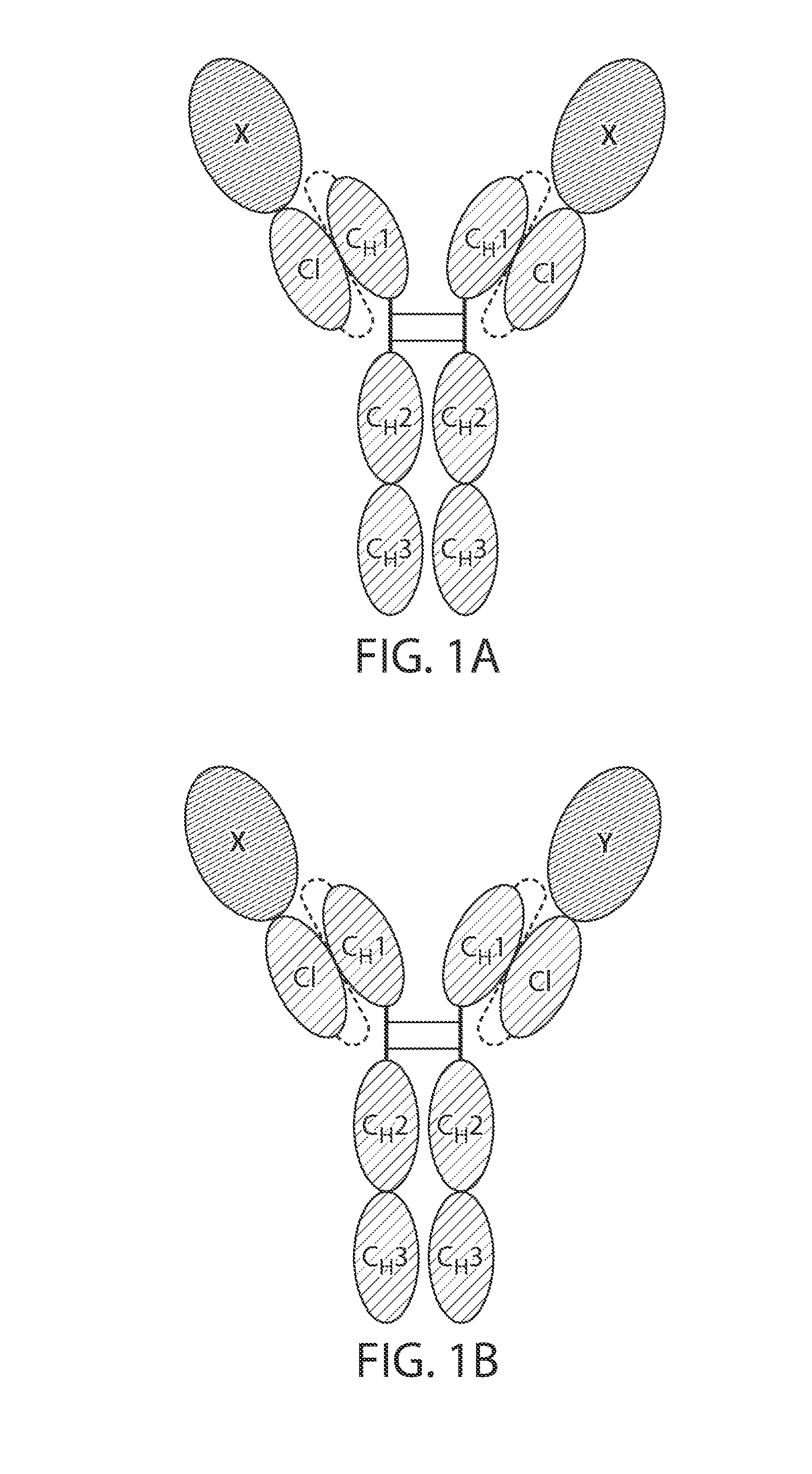
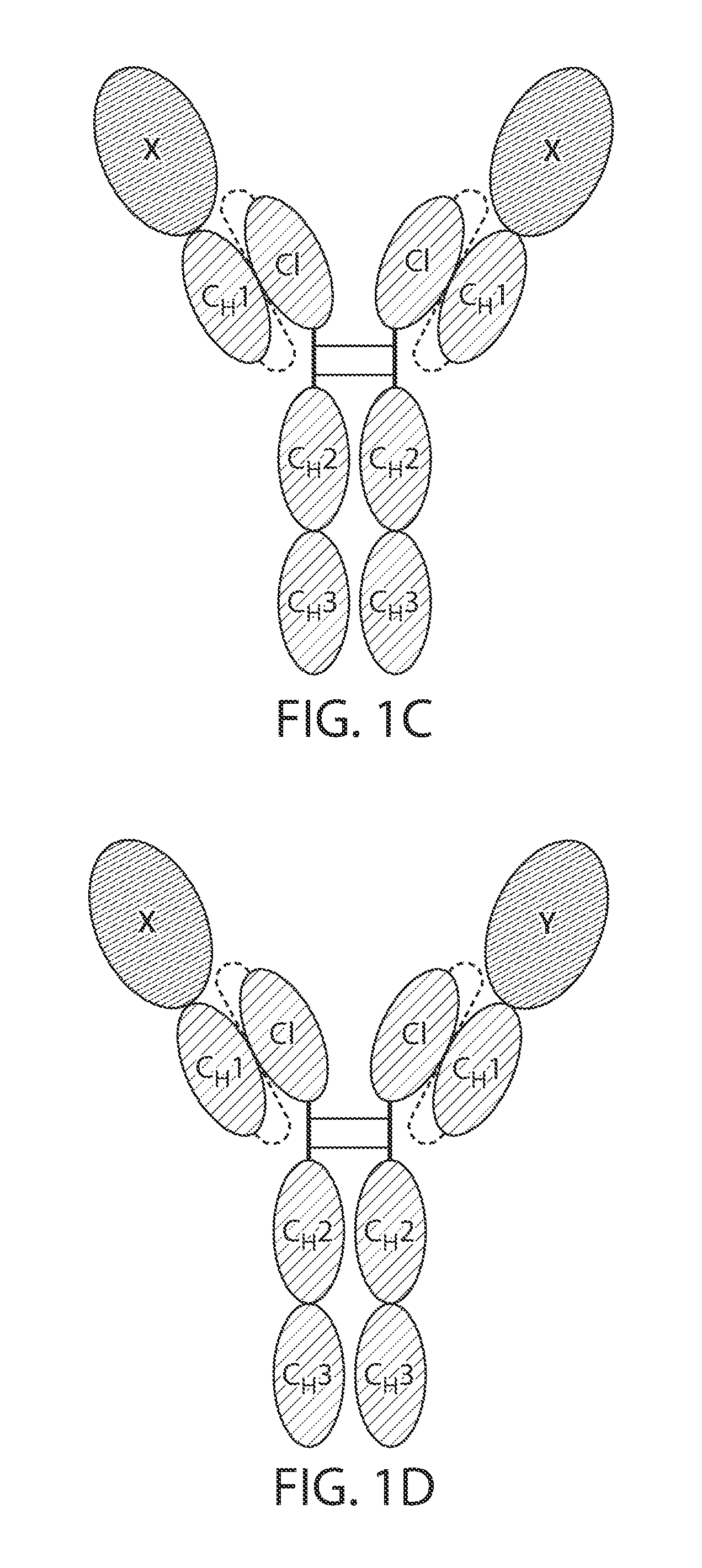
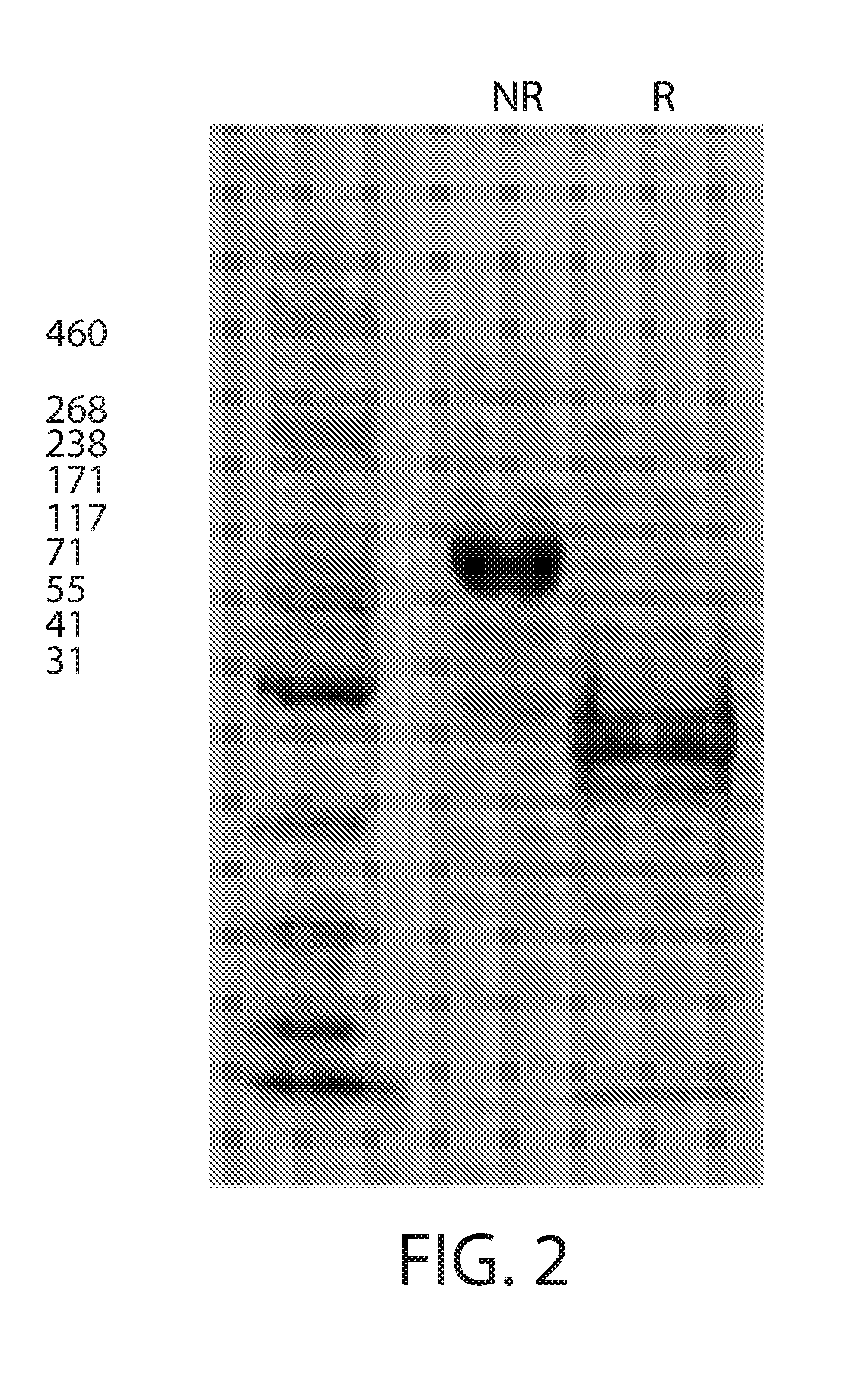
Single chain fc fusion proteins
a single-chain fusion protein and fusion protein technology, applied in the field of single-chain fc fusion proteins, can solve the problems of inefficient dimerization or reduction of the half-life of the dimer molecule, immunogenicity and poor pharmacokinetic properties, and loss of bioactivity, so as to improve the properties and improve the bioactivity. , the effect of favorable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0164]Design of Factor IX scCLCH1-Fc
[0165]The single chain factor IX molecule contains the factor IX sequence followed by a 10 residue linker having the amino acid sequence: GGGGSGGGGS (SEQ ID NO: 11), the CL domain of IgG1 followed by a 20 residue linker having the amino acid sequence: GGGGSGGGGSGGGGSGGGGS (SEQ ID NO: 12) followed by the CHL hinge and Fc portions of human IgG1.
Expression and Characterization of Factor IX scCLCH1-Fc
[0166]The gene, having the following DNA sequence:
(SEQ ID NO: 13)ATGTACCGGATGCAGCTGCTGAGCTGTATCGCCCTGTCTCTGGCCCTCGTGACCAACAGCACCGTGTTTCTGGACCACGAGAACGCCAACAAGATCCTGAACCGGCCCAAGCGGTACAACAGCGGCAAGCTGGAAGAGTTCGTGCAGGGCAACCTGGAACGCGAGTGCATGGAAGAGAAGTGCAGCTTCGAAGAGGCCAGAGAGGTGTTCGAGAACACCGAGCGGACCACCGAGTTCTGGAAGCAGTACGTGGACGGCGACCAGTGCGAGAGCAACCCCTGTCTGAATGGCGGCAGCTGCAAGGACGACATCAACAGCTACGAGTGCTGGTGCCCCTTCGGCTTCGAGGGCAAGAACTGCGAGCTGGACGTGACCTGCAACATCAAGAACGGCAGATGCGAGCAGTTCTGCAAGAACAGCGCCGACAACAAGGTCGTGTGCTCCTGCACCGAGGGCTACAGACTGGCCGAGAACCAGAAGTCCTGCG...
example 2
TNF-R2
[0170]Design of TNF-R2 scCLCH1-Fc
[0171]The single chain TNFR2 molecule contains the TNFR2 sequence followed by a 10 residue linker, GGGGSGGGGS (SEQ ID NO: 11), the CL domain of IgG1 followed by a 20 residue linker GGGGSGGGGSGGGGSGGGGS (SEQ ID NO: 12) followed by the CH1, hinge and Fc portions of human IgG1.
Expression of TNF-R2 scCLCH1-Fc
[0172]The gene, having the following DNA sequence:
(SEQ ID NO: 14)ATGTATAGGATGCAGCTCCTCAGCTGCATCGCTCTGTCCCTCGCCCTGGTGACCAACAGCCTCCCTGCCCAGGTGGCCTTTACACCCTACGCTCCTGAGCCCGGAAGCACCTGCAGGCTCAGGGAGTACTACGATCAGACCGCCCAAATGTGTTGCAGCAAGTGCTCCCCTGGCCAGCACGCCAAGGTGTTCTGCACCAAGACAAGCGATACCGTGTGCGATAGCTGTGAGGACAGCACCTACACCCAGCTGTGGAATTGGGTGCCCGAGTGCCTGAGCTGTGGCAGCAGGTGCAGCAGCGATCAGGTGGAGACACAGGCCTGCACCAGAGAGCAGAACAGGATTTGTACCTGCAGGCCCGGCTGGTATTGCGCCCTGAGCAAGCAGGAGGGATGTAGGCTGTGCGCCCCTCTGAGGAAATGCAGACCTGGCTTTGGAGTGGCTAGGCCCGGCACCGAGACATCCGACGTGGTGTGCAAGCCTTGTGCCCCTGGCACCTTTTCCAACACCACCAGCTCCACCGACATCTGCAGGCCCCATCAGATTTGCAACGTGGTGGCCATCCCCGGAAACGCTAGCATGGATGC...
example 3
IL1Ra
[0176]Design of IL1Ra scCLCH1-Fc
[0177]The single chain IL1Ra molecule contains the IL1Ra sequence followed by a 10 residue linker, GGGGSGGGGS (SEQ ID NO: 11), the CL domain of IgG1 followed by a 20 residue linker, GGGGSGGGGSGGGGSGGGGS (SEQ ID NO: 12) followed by the CH1, hinge and Fc portions of human IgG1.
Expression of IL1Ra scCLCH1-Fc
[0178]The gene having the following DNA sequence:
(SEQ ID NO: 15)ATGTACCGGATGCAGCTGCTGTCCTGTATCGCCCTGTCTCTGGCCCTGGTCACCAACTCCAGACCCTCTGGCCGGAAGTCCTCCAAGATGCAGGCCTTCCGGATCTGGGACGTGAACCAGAAAACCTTCTACCTGCGGAACAACCAGCTGGTGGCCGGCTATCTGCAGGGCCCCAACGTGAACCTGGAAGAGAAGATCGACGTGGTGCCCATCGAGCCCCACGCCCTGTTTCTGGGAATCCACGGCGGCAAGATGTGCCTGTCCTGCGTGAAGTCCGGCGACGAGACACGGCTGCAGCTGGAAGCCGTGAACATCACCGACCTGTCCGAGAACCGGAAGCAGGACAAGAGATTCGCCTTCATCAGATCCGACTCCGGCCCTACCACCTCCTTCGAGTCTGCTGCTTGCCCCGGCTGGTTCCTGTGCACCGCCATGGAAGCTGACCAGCCCGTGTCCCTGACCAACATGCCTGACGAGGGCGTGATGGTCACCAAGTTCTATTTTCAGGAAGATGAGGGCGGAGGCGGCTCTGGCGGCGGAGGATCTAGAACAGTGGCCGCTCCCTCCGTGTTCATCTTCCCACCTTCCGA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com