Cll1-specific multi-chain chimeric antigen receptor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Multi-Chain CARs
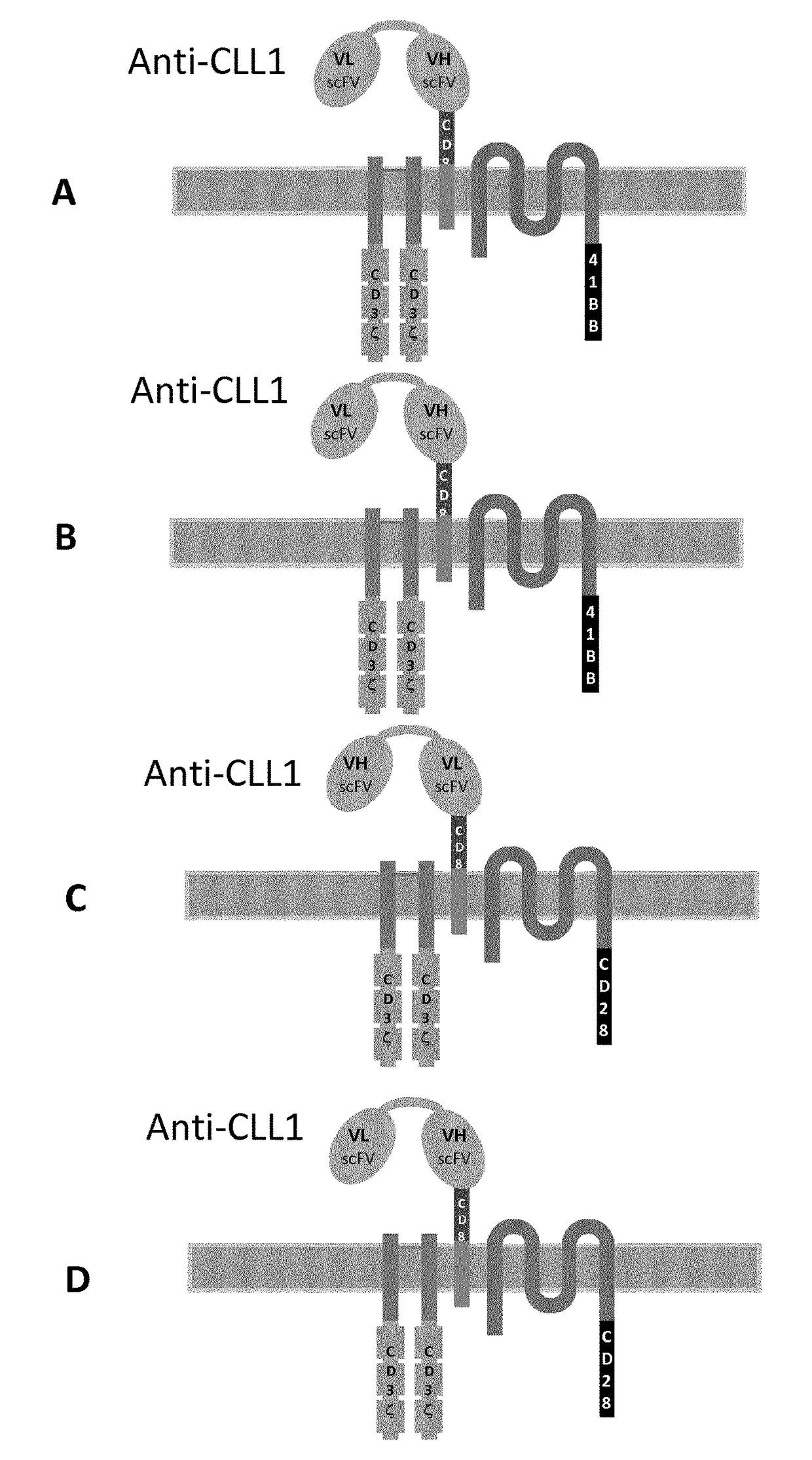
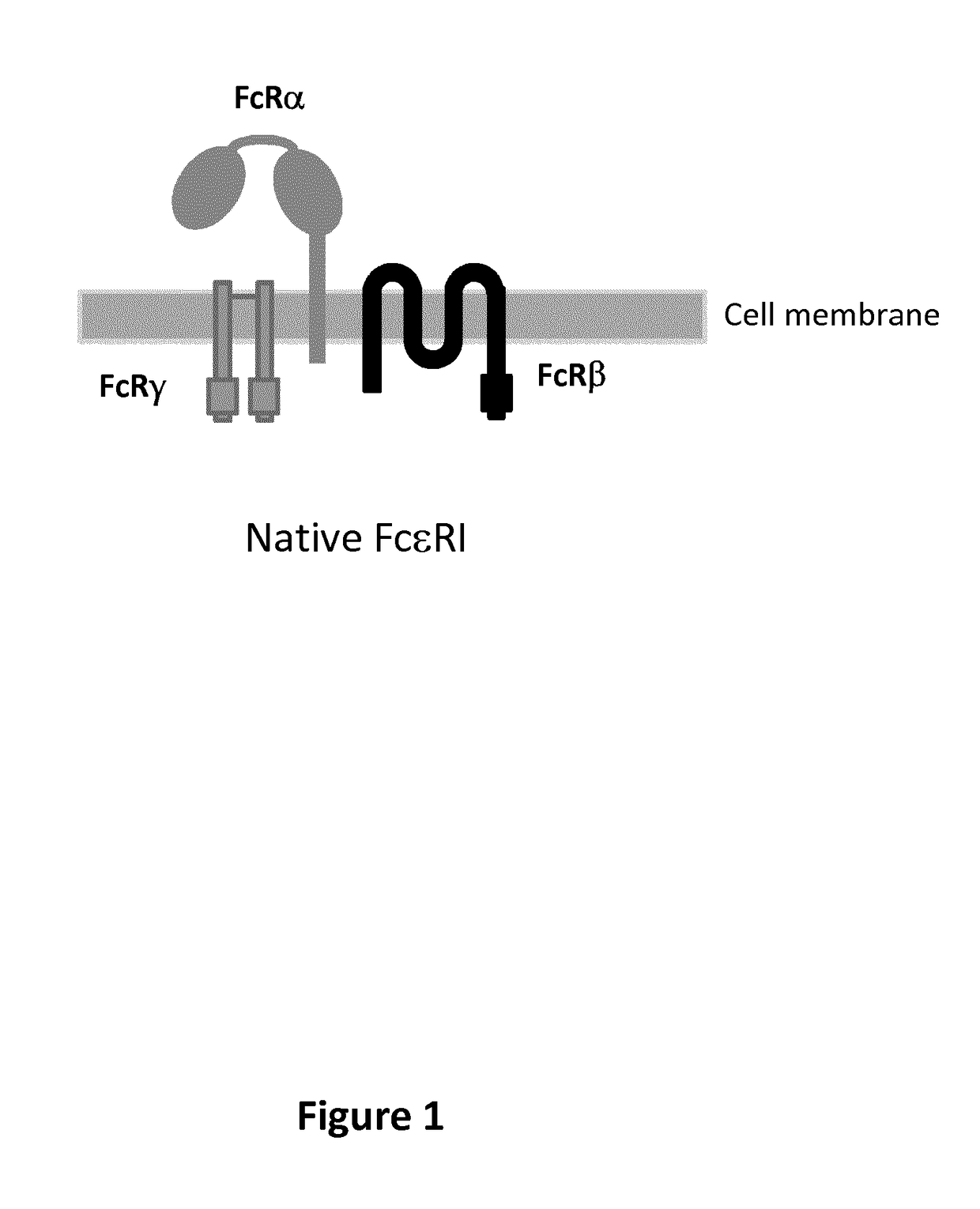
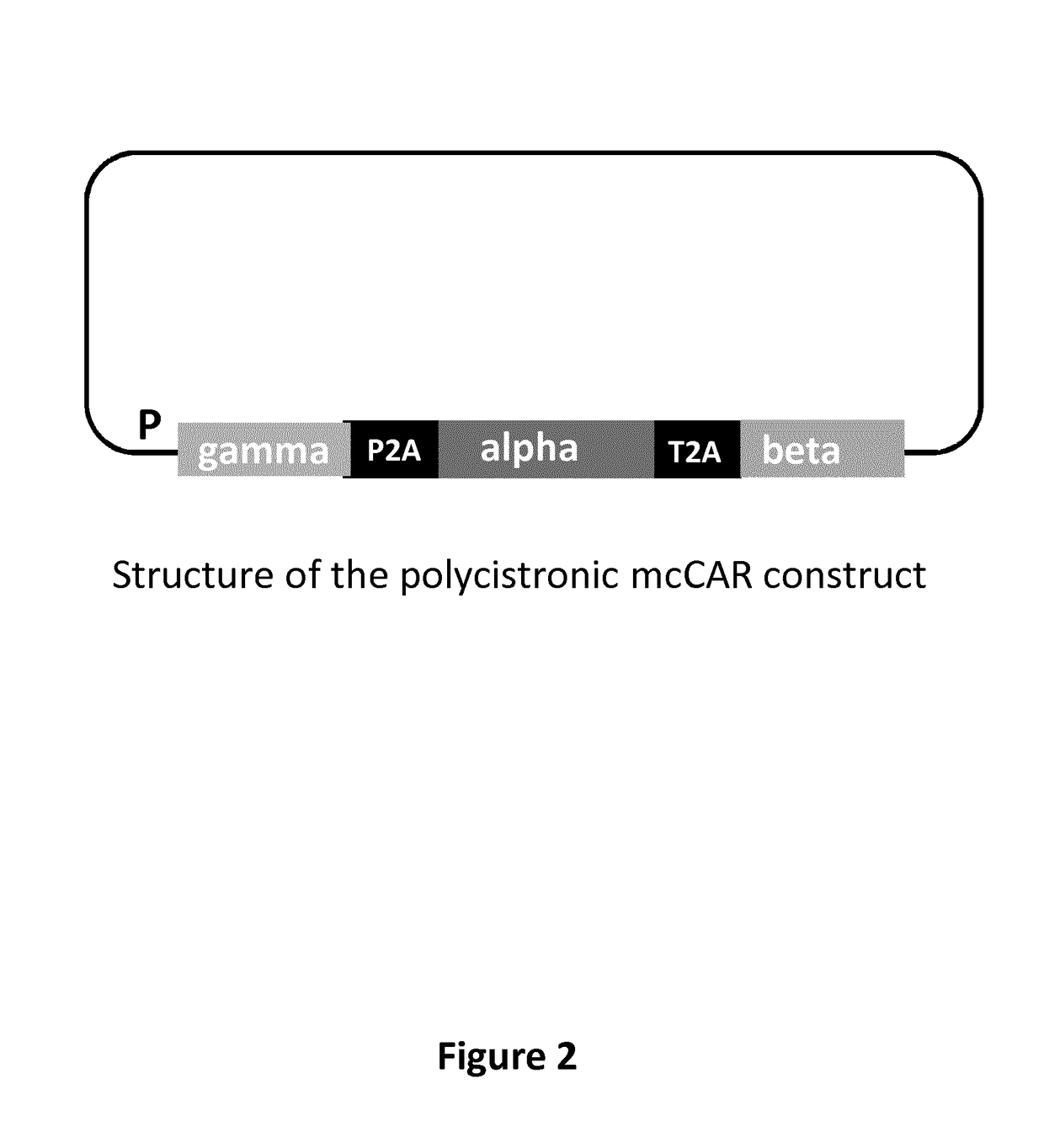
[0674]Multi-chain CARs targeting the CLL1 antigen were designed based on the high affinity receptor for IgE (FcεRI) such as depicted in FIG. 2 to FIG. 4. The FcεRI expressed on mast cells and basophiles triggers allergic reactions. It is a tetrameric complex composed of a single a subunit, a single β subunit and two disulfide-linked γ subunits. The a subunit contains the IgE-binding domain. The β and γ subunits contain ITAMs that mediate signal transduction. In every multi-chain CAR, the extracellular domain of the FcRα chain was deleted and replaced by the respective scFv referred to μ ln Table 5 respectively and the CD8α hinge (SEQ ID NO: 2) and the ITAM of the FcRβ chain and / or the FcRγ chain was deleted. The resulting constructions had the structure detailed in table 6.
example 2
n of Anti-CLL1 mcCARs in Human T Cells
[0675]Primary T-Cell Cultures
[0676]T cells were purified from Buffy coat samples provided by EFS (Etablissement Francais du Sang, Paris, France) using Ficoll gradient density medium (Ficoll Paque PLUS / GE Healthcare Life Sciences). The PBMC layer was recovered and T cells were purified using a commercially available T-cell enrichment kit (Stem Cell Technologies). Purified T cells were activated in X-Vivo™-15 medium (Lonza) supplemented with 20 ng / mL Human IL-2 (Miltenyi Biotech), 5% Human Serum (Sera Laboratories), and Dynabeads Human T activator CD3 / CD28 at a bead:cell ratio 1:1 (Life Technologies). After activation cells were grown and maintained in X-Vivo™-15 medium (Lonza) supplemented with 20 ng / mL Human IL-2 (Miltenyi Biotec) and 5% Human Serum (Sera Laboratories).
[0677]Models of AML and Clorofarabine, Fludarabine or Cytarabine Resistant AML
[0678]Originally, an AML-positive cell line, such as MOLM13 cell line, has been established from the ...
example 3
tion of T Cells Transiently Expressing the Anti-CLL1 mcCARs Following Coculture with Target Cells
[0688]24 hours post electroporation, human T cells engineered using polycistronic mRNAs encoding the multi-chain CARs were co-cultured with target (Daudi) or AML cell line control cells for 6 hours. The CD8+ T cells were then analyzed by flow cytometry to detect the expression of the degranulation marker CD107a at their surface. This experiment aims to check that the human CD8+ T cells expressing the CLL1 multi-chain CARs degranulate in coculture with CLL1 expressing target cells but not in coculture with control cells.
[0689]Degranulation Assay (CD107a Mobilization)
[0690]T-cells were incubated in 96-well plates (40,000 cells / well), together with an equal amount of cells expressing or not the CLL1 protein. Co-cultures were maintained in a final volume of 100 μl of X-Vivo™-15 medium (Lonza) for 6 hours at 37° C. with 5% CO2. CD107a staining was done during cell stimulation, by the addition...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com