Attenuated Infectious Bronchitis Virus
a coronavirus and infectious bronchitis technology, applied in the field of attenuated coronavirus, can solve the problems of reduced egg production and quality, and death of kidney diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of an IBV Reverse Genetics System Based on M41-CK
[0245]A M41-CK full-length cDNA was produced by replacement of the Beaudette cDNA in the Vaccinia virus reverse genetics system previously described in PCT / GB2010 / 001293 (herein incorporated by reference) with synthetic cDNA derived from the M41 consensus sequence.
[0246]The IBV cDNA within recombinant Vaccinia virus (rVV) rVV-BeauR-Rep-M41 Struct described in Armesto, Cavanagh and Britton (2009). PLoS ONE 4(10): e7384. doi:10.1371 / journal.pone.0007384, which consisted of the replicase derived from IBV Beaudette strain and the structural and accessory genes and 3′ UTR from IBV M41-CK, was further modified by replacement of the Beaudette 5′ UTR-Nsp2-Nsp3 sequence with the corresponding sequence from IBV M41-CK. The resulting IBV cDNA consisted of 5′ UTR-Nsp2-Nsp3 from M41, Nsp4-Nsp16 from Beaudette and the structural and accessory genes and 3′ UTR from M41. This cDNA was further modified by the deletion of the Beaudette Nsp4-Nsp16 seq...
example 2
ng the Pathogenicity of Recombinant M41 Viruses
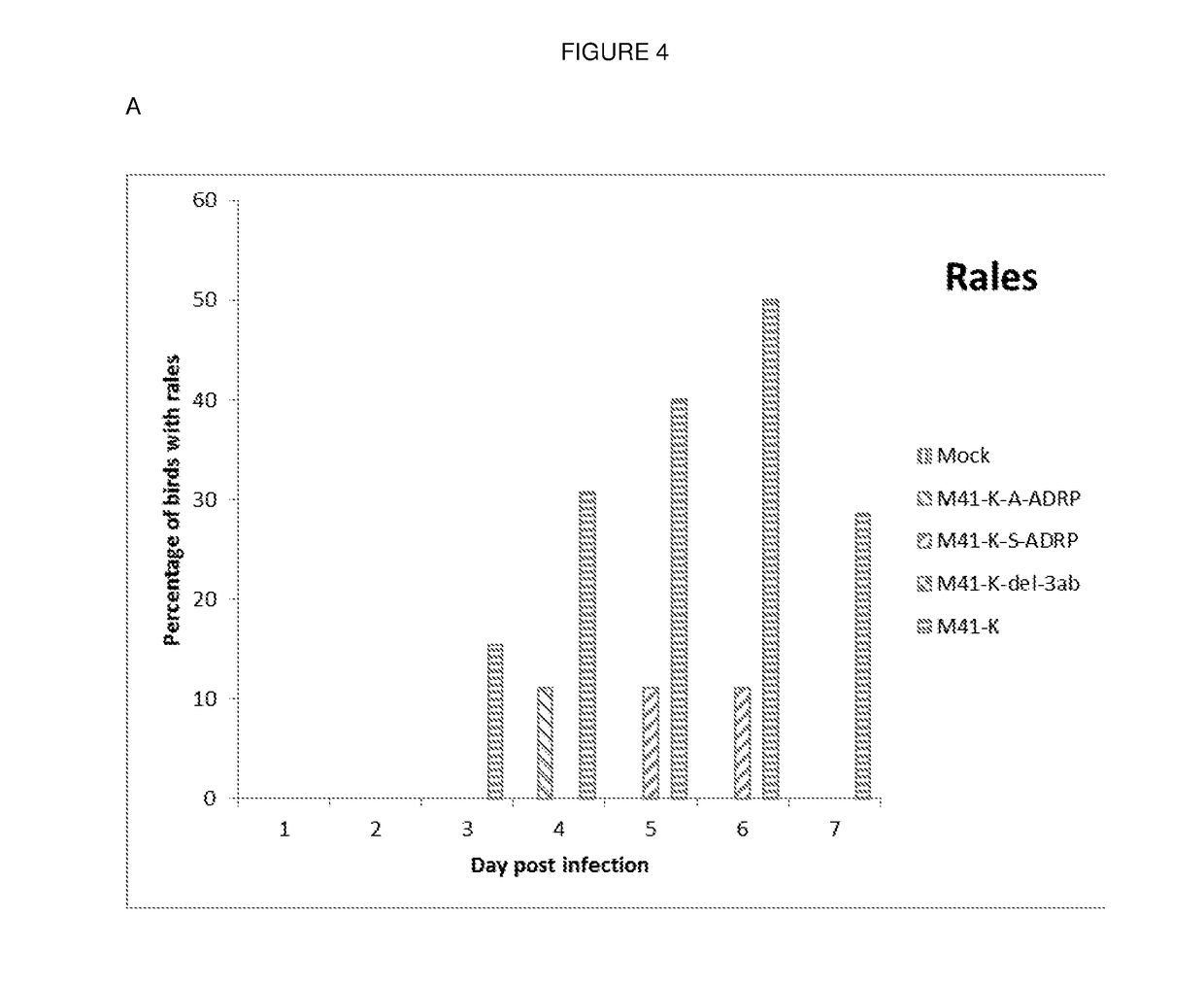
[0247]Three recombinants were produced using the reverse genetics system described in Example 1. These recombinants were named rIBV M41K-S-ADRP, rIBV M41K-A-ADRP and rIBV M41K-del3ab.
[0248]The three recombinants were shown to grow in a similar manner in chicken kidney cells as wild-type M41-CK / M41-K (FIGS. 7 and 8).
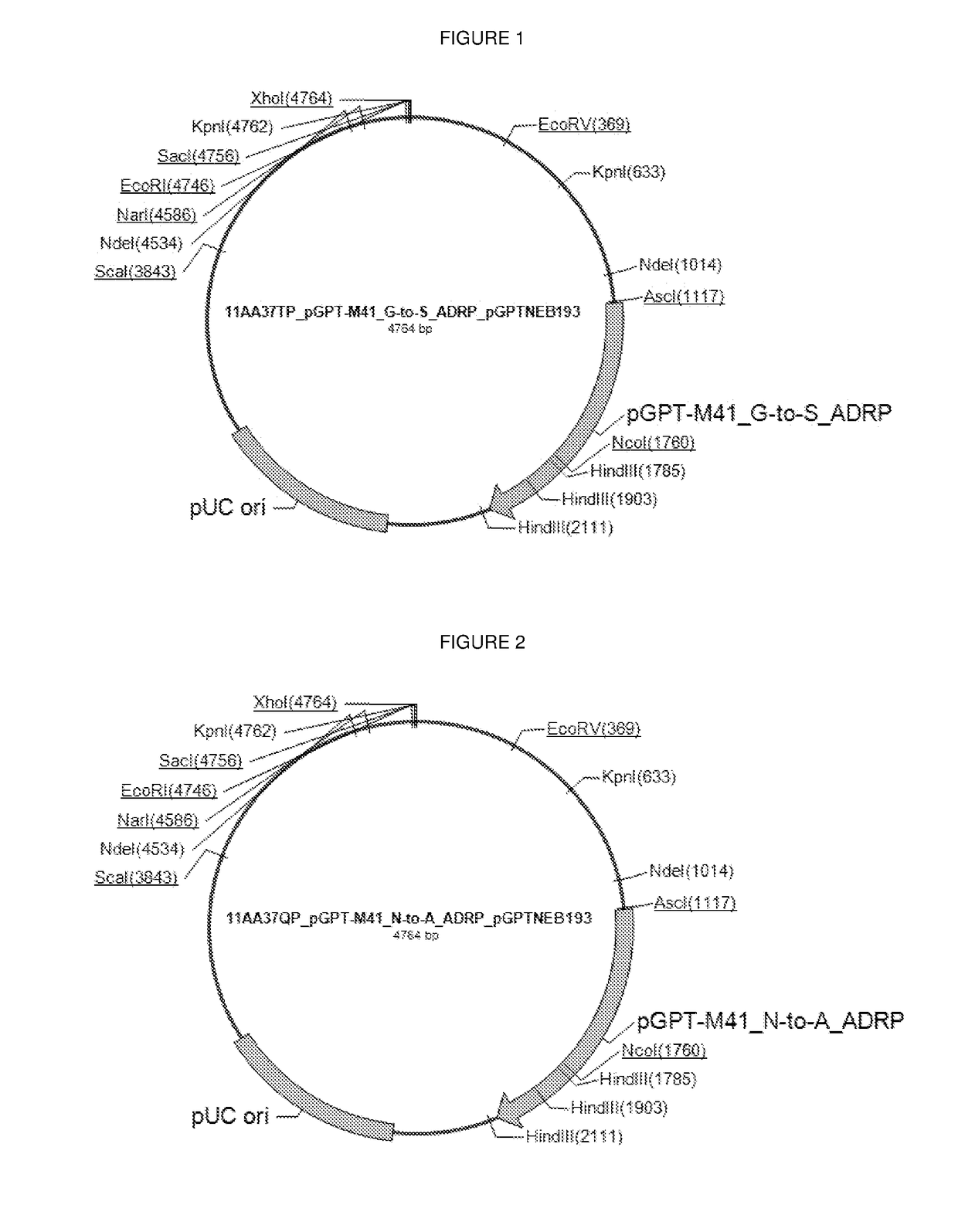
[0249]rIBV M41K-S-ADRP has a mutation at nucleotide position G3685A in the adenosine diphosphate-ribose-1′-phosphatase (ADRP) region of nsp-3 resulting in an amino acid mutation from G to S. This mutation was introduced into the rVV containing M41-K using the plasmid shown in FIG. 1.
[0250]rIBV M41K-A-ADRP has two mutations at nucleotide positions A3664G and A3665C in the ADRP region of nsp-3 resulting in an amino acid mutation from N to A. This mutation was introduced into the rVV containing M41-K using the plasmid shown in FIG. 2.
[0251]rIBV M41K-del3ab has a deletion from nucleotide position 23867 to 24214 in gene 3 resultin...
example 3
on / Challenge Study with M41-R
[0253]Candidate vaccine viruses are tested in studies in which fertilized chicken eggs are vaccinated in ovo at 19 days embryonation and in which the hatchability of the inoculated eggs is determined. The clinical health of the chickens is investigated and the chickens are challenged at 21 days of age with a virulent IB M41 challenge virus at 103.65 EID50 per dose.
[0254]Clinical signs are investigated after challenge protection by the vaccine and a ciliostasis test is performed at 5 days after challenge to investigate the effect of the challenge viruses on movement of the cilia and protection by the vaccine against ciliostasis (inhibition of cilia movement).
TABLE 3Betweenhatch andEnd ofTreatmentEggsAt hatchchallengeChallengestudySalineMDA−DetermineClinicalChickens5 days postIB M41-MDA−hatchexaminationat age ofchallenge aK-A-percent-of birds21 daysciliostasisADRPagestest will beIB M41-MDA−doneK-S-ADRPIB M41-MDA−K-3abIn ovo vaccination of 19 days embryonat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
| genomic size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com