Molten metal rechargeable electrochemical cell
a rechargeable electrochemical cell and molten metal technology, applied in the direction of cell components, electrochemical generators, jackets/cases materials, etc., can solve the problems of complex and inefficient cell configuration, effective sealing and anti-corrosion measures, and many significant technical drawbacks of traditional molten electrode electrochemical cells, so as to reduce cost, reduce chemical reactivity, and high commercial availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
hode Cells
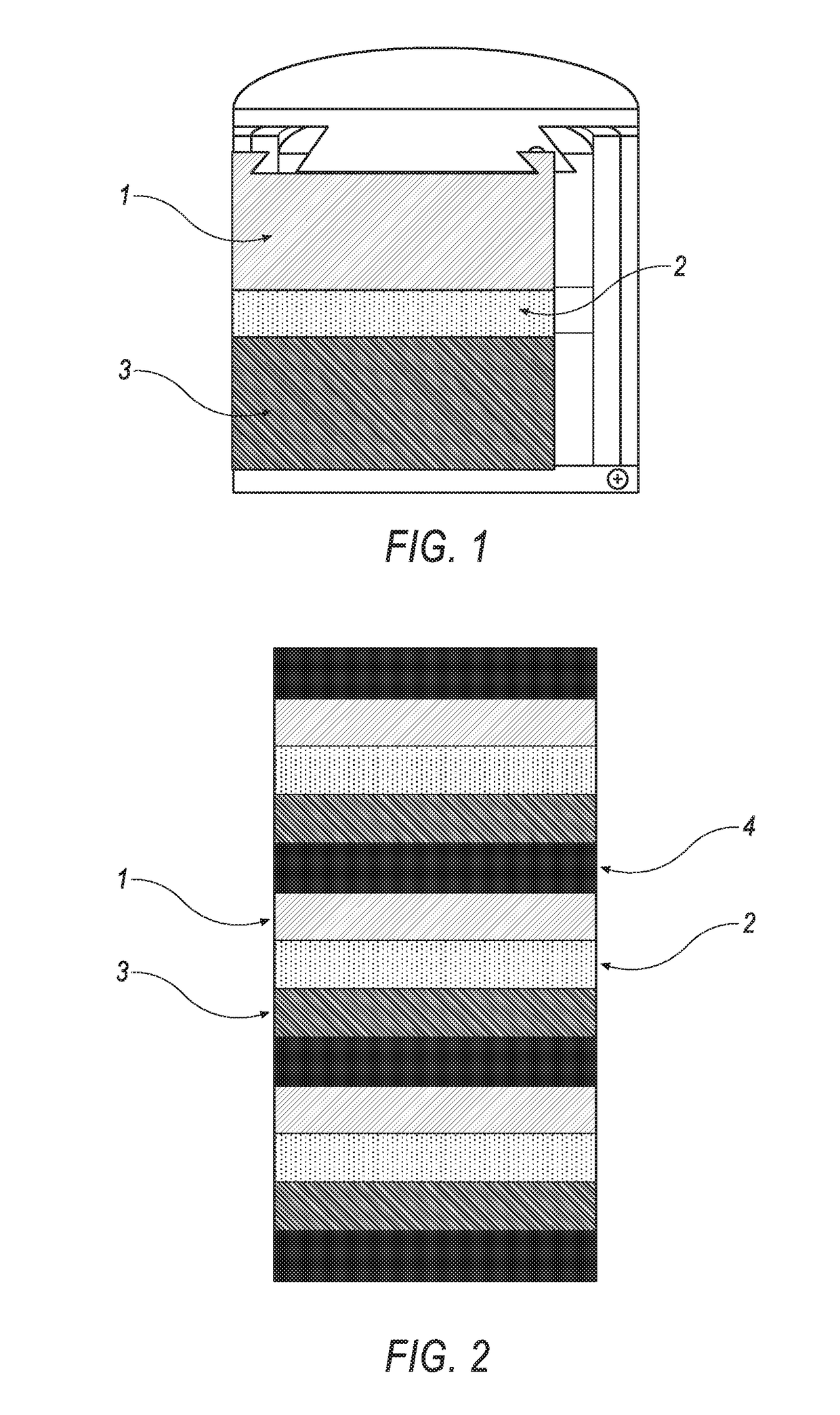
[0152]Five cells according to the present invention were formed using a Kerr Auto Electro-Melt furnace that was modified to include two holes in the cover to allow for the insertion of current collectors into the anode and cathode. The Kerr Auto Electro-Melt furnace included a graphite crucible placed on the inside of the furnace. Within the graphite crucible, an alumina crucible (33 mm outer diameter, 25 mm inner diameter, 105 mm length—McDanel Ceramics) was placed. The materials used for the molten metal battery were placed inside of the alumina crucible. 10 g of the cathode material (50 / 50 weight mixture of bismuth and tin granules (1-2 mm)) was first placed in the alumina crucible. 15 g of the electrolyte (a powder combination of 18% wt NaF, 48% wt AlF3, 16% wt CaF2, and 18% wt BaF2 that has already been mixed, heated to melting in alumina crucible using a muffle furnace at 900° C., then allowed to cool and solidify) was then placed in the alumina crucible. Then, 10 g ...
example 2
[0205]An example cell of the present invention was constructed according to the procedures described in Example 1, using 10 g of tungsten oxide (WO3) for the cathode material.
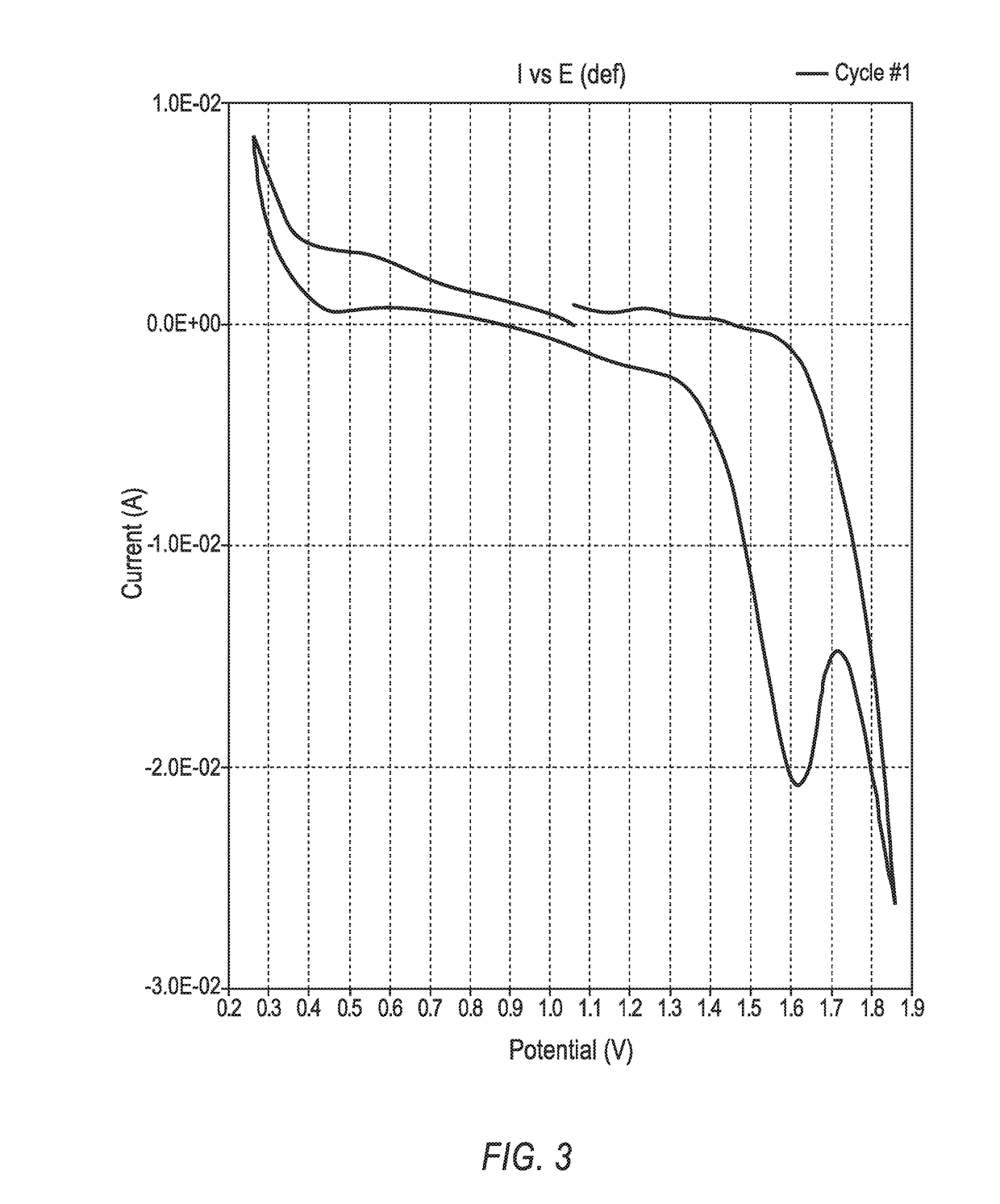
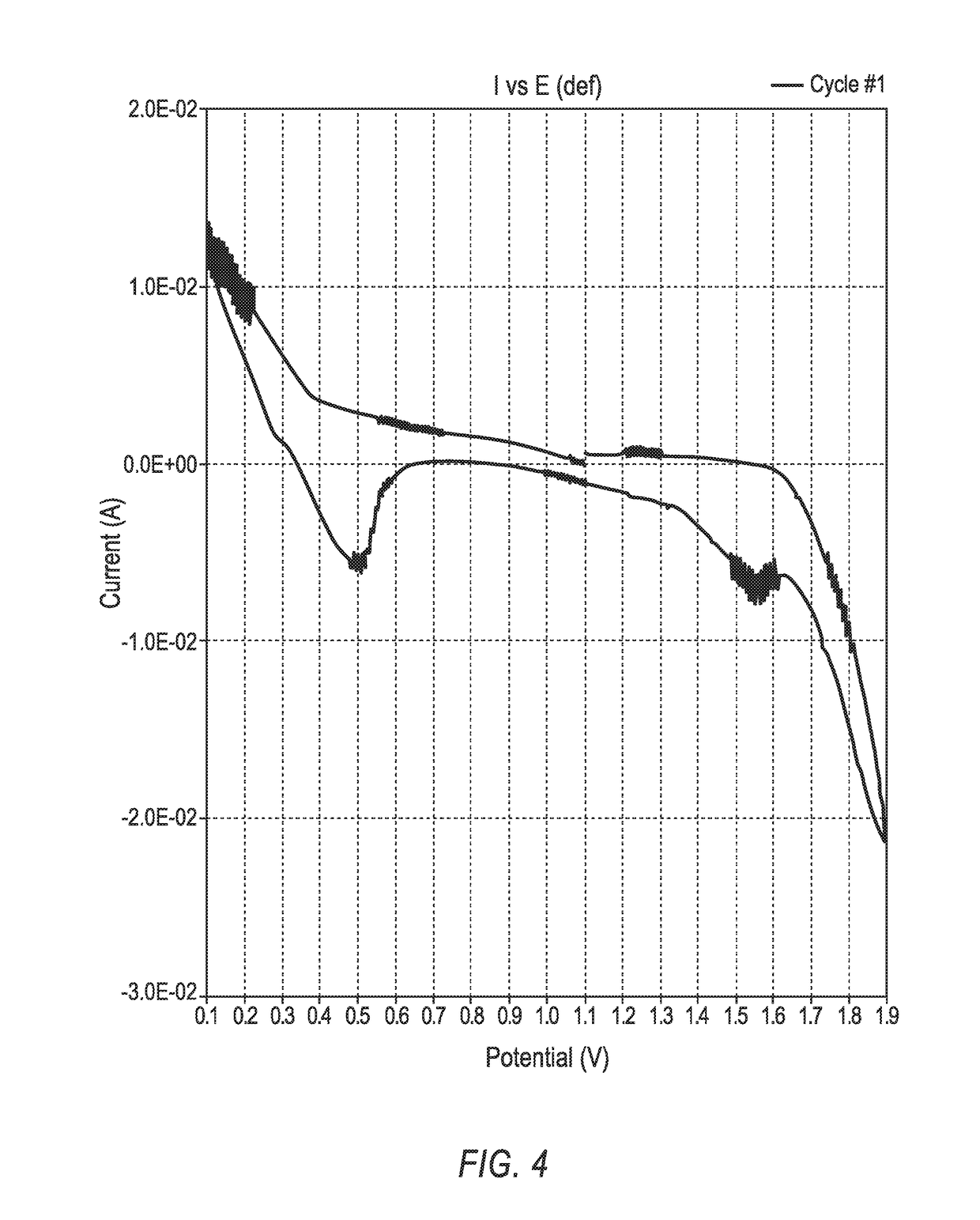
[0206]In the CV scan, i.e., the I vs. E plot, depicted in FIG. 9, the scan potentials ranged from 0.5 V (discharge voltage) to 1.8 V. This cell generated peak discharge currents at 0.5 V that were >9 mA / cm2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltages | aaaaa | aaaaa |
| operating temperatures | aaaaa | aaaaa |
| operating temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com