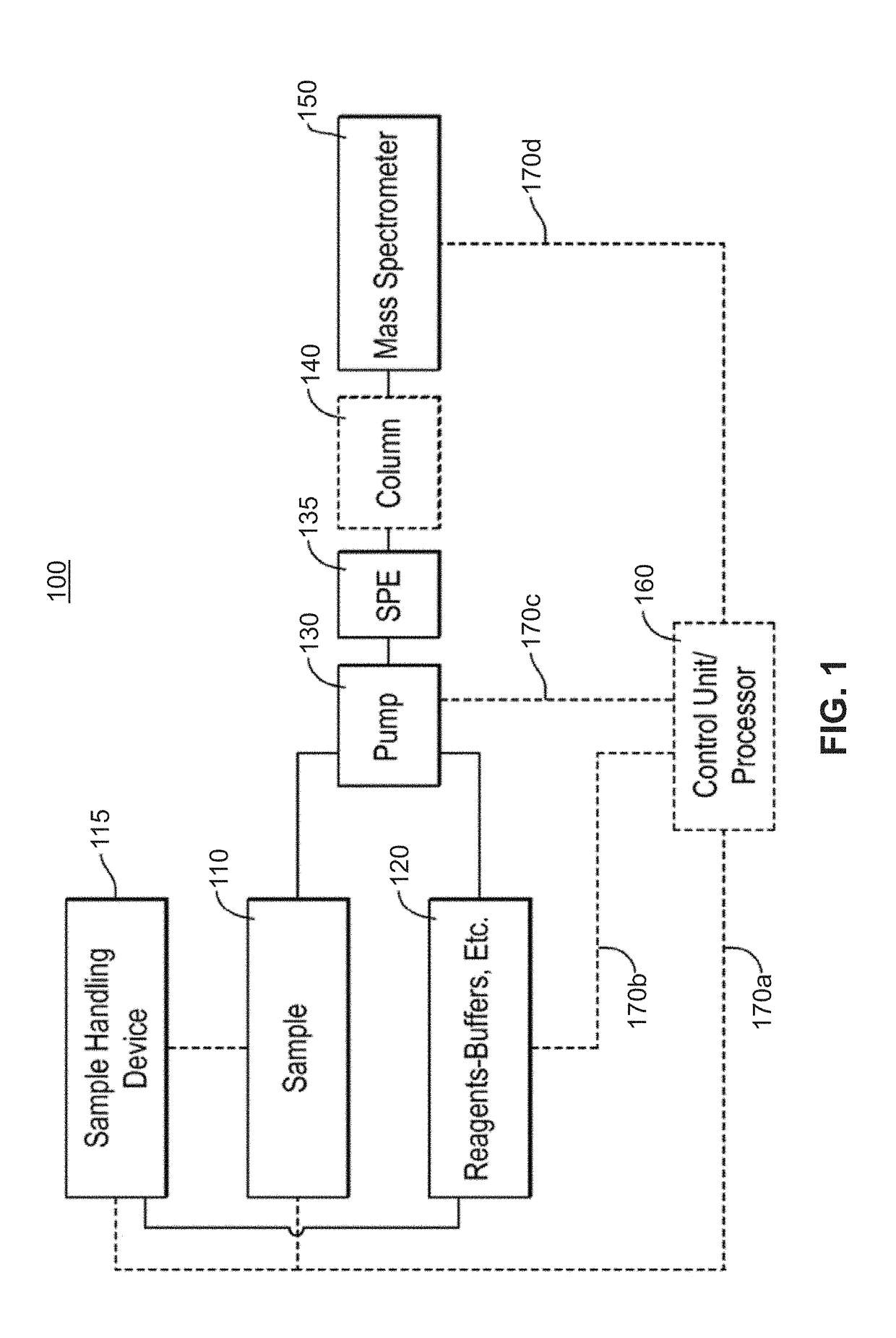
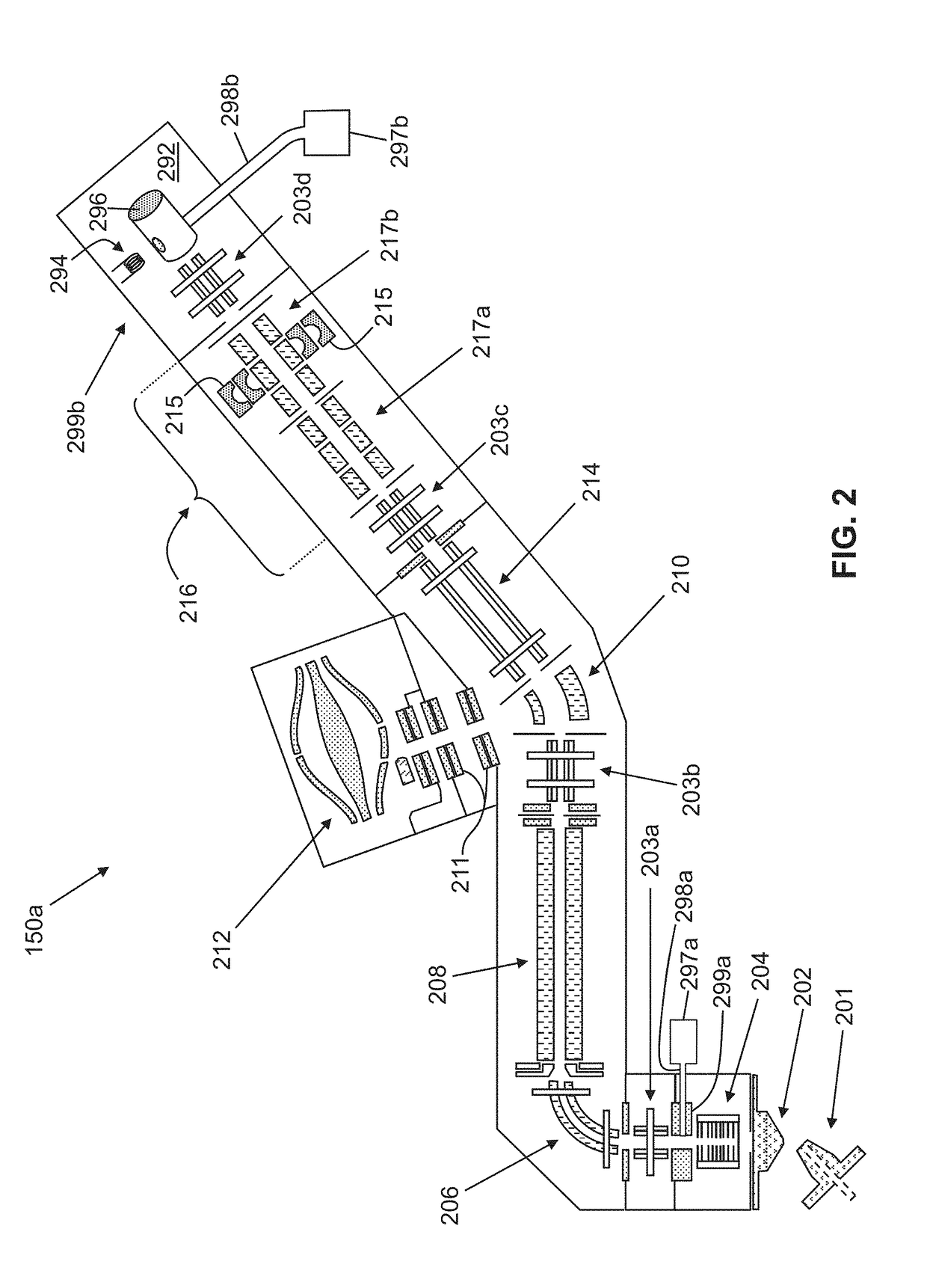
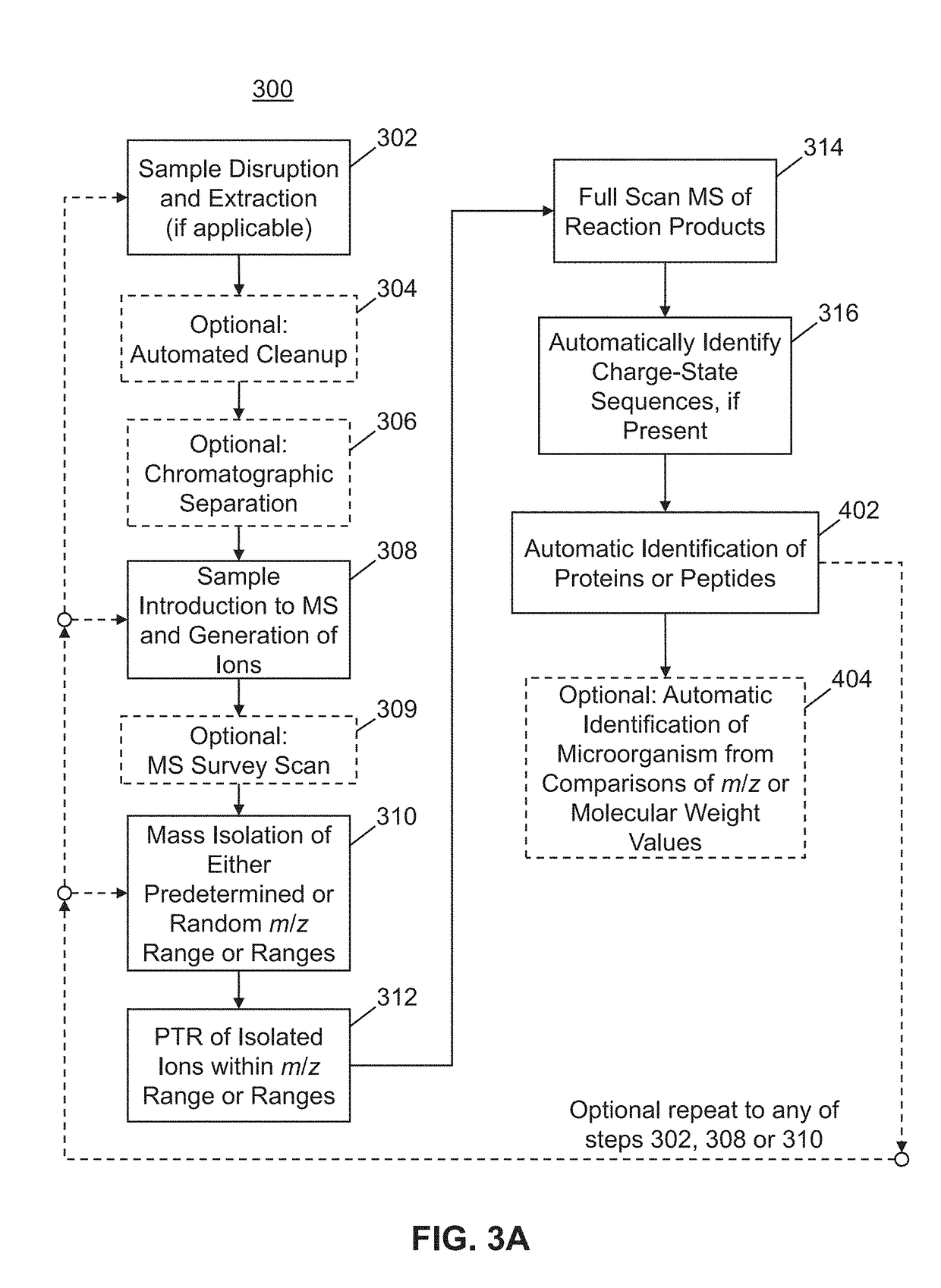
Methods for Top-Down Multiplexed Mass Spectral Analysis of Mixtures of Proteins or Polypeptides
a technology of protein and polypeptide mixtures, applied in the field of mass spectrometry, can solve the problems of limited application range of maldi-tof methods to pathogen characterization and identification, limited range of applications of maldi-tof methods, and inability to identify closely related microorganisms, etc., and achieve the effect of simplifying the mass spectrometric analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example a
[0154]FIGS. 4A and 4B provide an example of mass spectroscopic signal enhancement provided by a single PTR reaction step (e.g., as in the method 300 shown in FIG. 3A). In a first application (FIGS. 4A, 4B), an extract from the pathogen E. coli was analyzed via direct infusion; the mass spectrum of the first-generation electrospray-generated ions is shown in FIG. 4A. As expected, there are many proteins present that overlap at various m / z values leading to the presence of a broad spectral region between approximately m / z=780 and m / z=1420 within which many ions are detected but with very little usable information in terms of discernible protein charge state distributions. Next, an m / z “window” of the first-generation ions of width 2 Th and centered at m / z=750 was isolated and the resulting isolated ion population was subjected to PTR reaction. The m / z position 412a shown in FIG. 4A indicates the center position of the isolation window.
[0155]FIG. 4B shows a mass spectrum of the PTR rea...
example b
[0156]FIGS. 5A and 5B illustrate an example of analysis of an E. Coli extract that is performed by a procedure that includes two stages of PTR reaction (for example, see steps 327, 328, 329 and 330 of method 380 in FIG. 3C). FIG. 5A illustrates a PTR product ion spectrum generated isolated first-generation precursor ions from within a 5 Th mass window centered at m / z=1200, indicated by position 711 in FIG. 5A. In this instance, the initial PTR spectrum does not include peaks that are sufficiently well resolved to enable identification of any proteins in the sample. Therefore, a subset of the first-generation PTR product ions were isolated for a second stage of PTR from within a 5 Th mass window centered at m / z=1320, indicated by position 712a in FIG. 5A and position 712b in FIG. 5B. The second-generation PTR product ions, which occur at m / z ratios greater than 1320 in FIG. 5B show clear charge-state distribution patterns that may be successfully used for identification of proteins i...
example c
[0158]As should be evident from the previous discussions, positive ion electrospray ionization of any protein or polypeptide molecule will produce a plurality of ions comprising different respective charge states (i.e., number of charges) as a result of different degrees of protonation of the original molecule. Charge states of +50 or more or possible and each charge state will be represented by multiple mass spectral lines representing different degrees of natural isotopic substitution. A further complication arises from the fact that for most natural biological samples, numerous different proteins of polypeptide molecules may be represented in a mass spectrum. A yet further complication arises from the fact that many other molecules—not necessarily of interest—may be present in a sample.
[0159]In many basic-research-oriented studies, the above-noted complicating factors of multiple analytes and multiple interfering species may be partially or wholly resolved by performing chromatic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com