Optogenetic System and Method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
endent Modulation of In Vitro Epileptic Activity Using Closed-Loop Optogenetic Stimulation
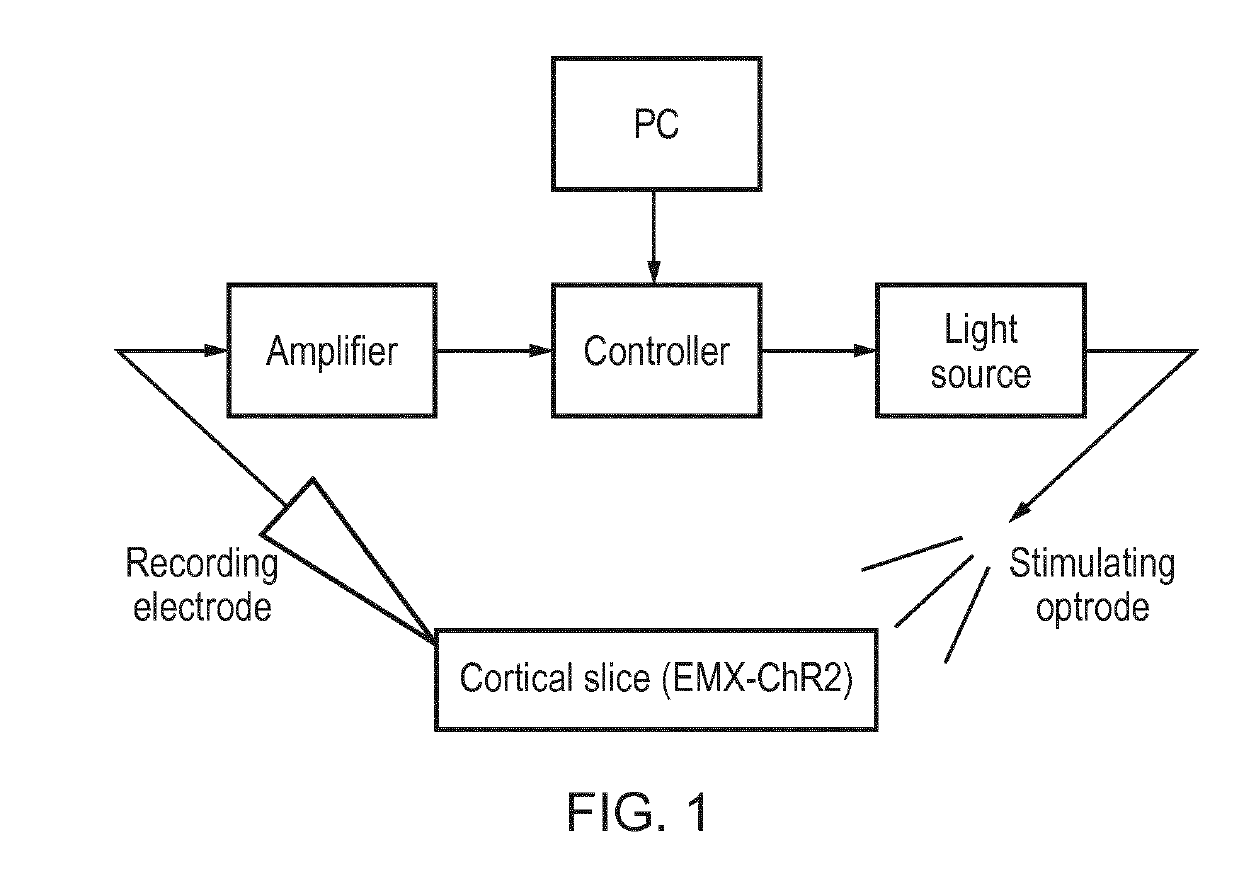
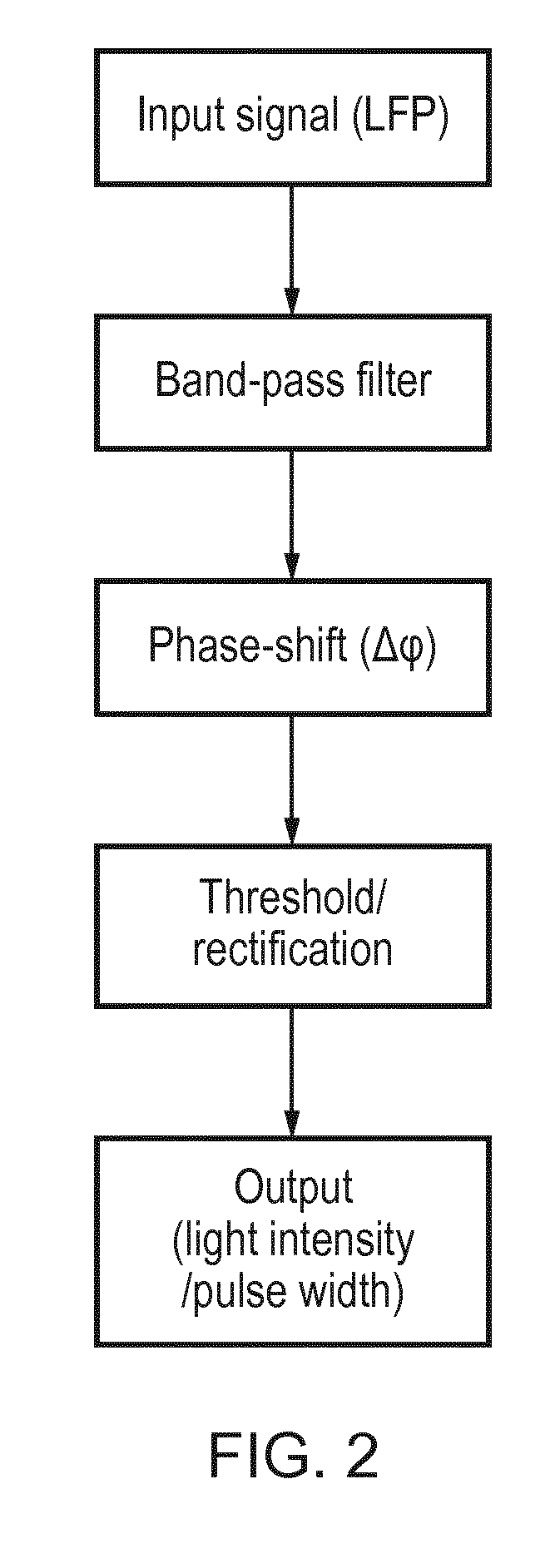
[0097]This example considers phase-dependent modulation of epileptic activity using closed-loop optogenetics in rodent brain slices selectively expressing Channelrhodopsins-2 (ChR2) either in excitatory pyramidal neurons using an Emx1 promoter, or in a subset of inhibitory cells using the parvalbumin (PV) promoter.
Experimental Details
[0098]Brain Slice Preparation
[0099]Coronal neocortical brain slices (400 μm) were prepared from Emx1-ChR2 and PV-ChR2 mice, which provides selective neuronal expression of channelrhodopsin-2 in glutamatergic cells (Gorski et al., 20021). The mice were perfused using the same ice-cold oxygenated (95% O2 / 5% CO2) sucrose-containing artificial cerebrospinal fluid (sACSF) used for cutting the brain slices; (sACSF in mm: 252 Sucrose, 24 NaHCO3, 2 MgSO4, 2 CaCl2), 10 glucose, 3.5 KCl, 1.25 NaH2PO4). Rodent brain slices were cut using a 5100 mz vibratome (Camden Instrument...
example 2
endent Modulation of in Silico Epileptic Activity Using Closed-Loop Stimulation
[0121]This example considers computational modelling work that parallels the in vitro closed-loop optogenetic stimulation experiments discussed above.
[0122]Methods
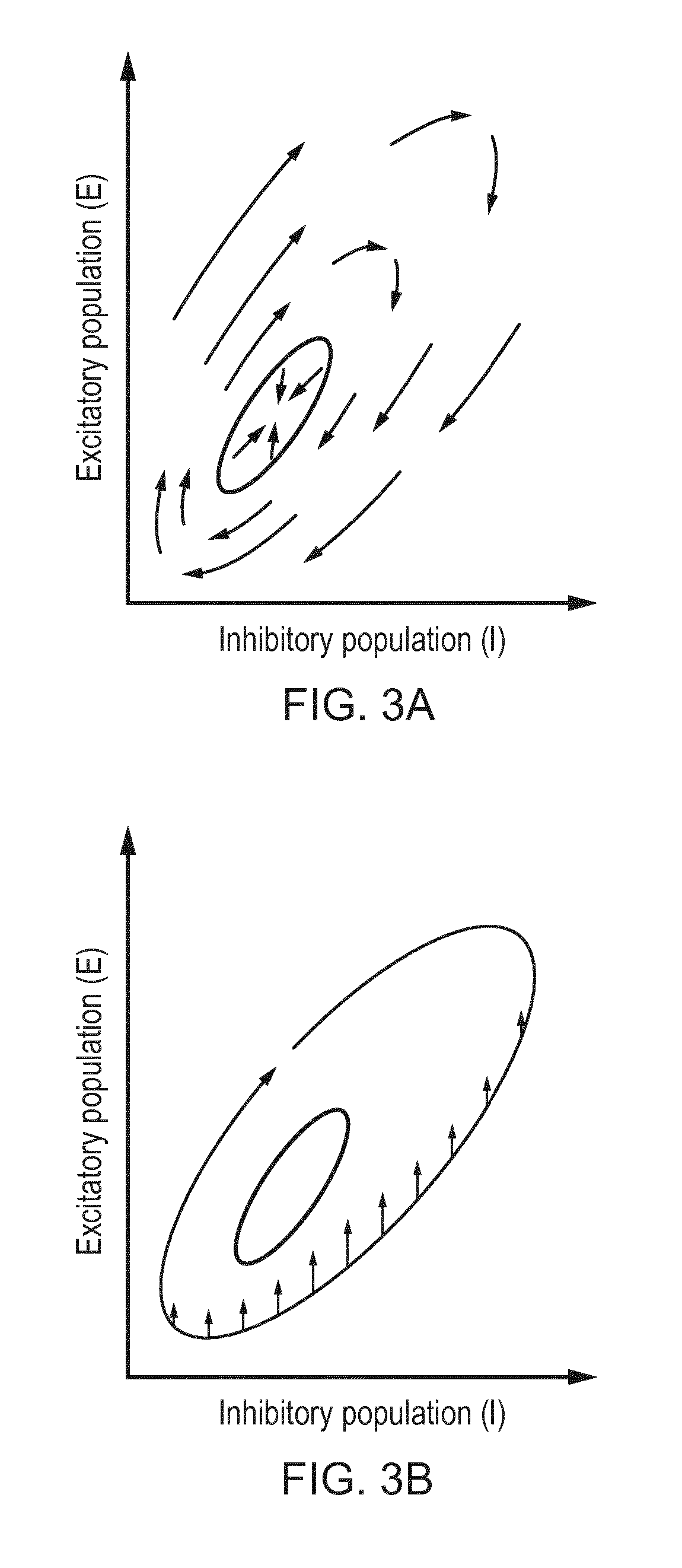
[0123]Modelling Epileptiform Activity
[0124]The model used here is a variant of the classic Wilson-Cowan neural population model [Wilson and Cowan, 19723], which is described in detail in previous publications [Wang et al., 2012, Wang et al., 20144]. The two-variable version of it is used, which models the neural tissue as a single excitatory population and a single inhibitory population. This model is able to capture epileptiform spikes and epileptiform discharges [Wang et al., 20125], which are the two key activity types from the experimental data. 3Wilson, H. and Cowan, J. (1972). Excitatory and inhibitory interactions in localized populations of model neurons. Biophysical Journal, 12(1):1-244Wang, Y., Goodfellow, M., Taylor, P. N., and Baier,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com