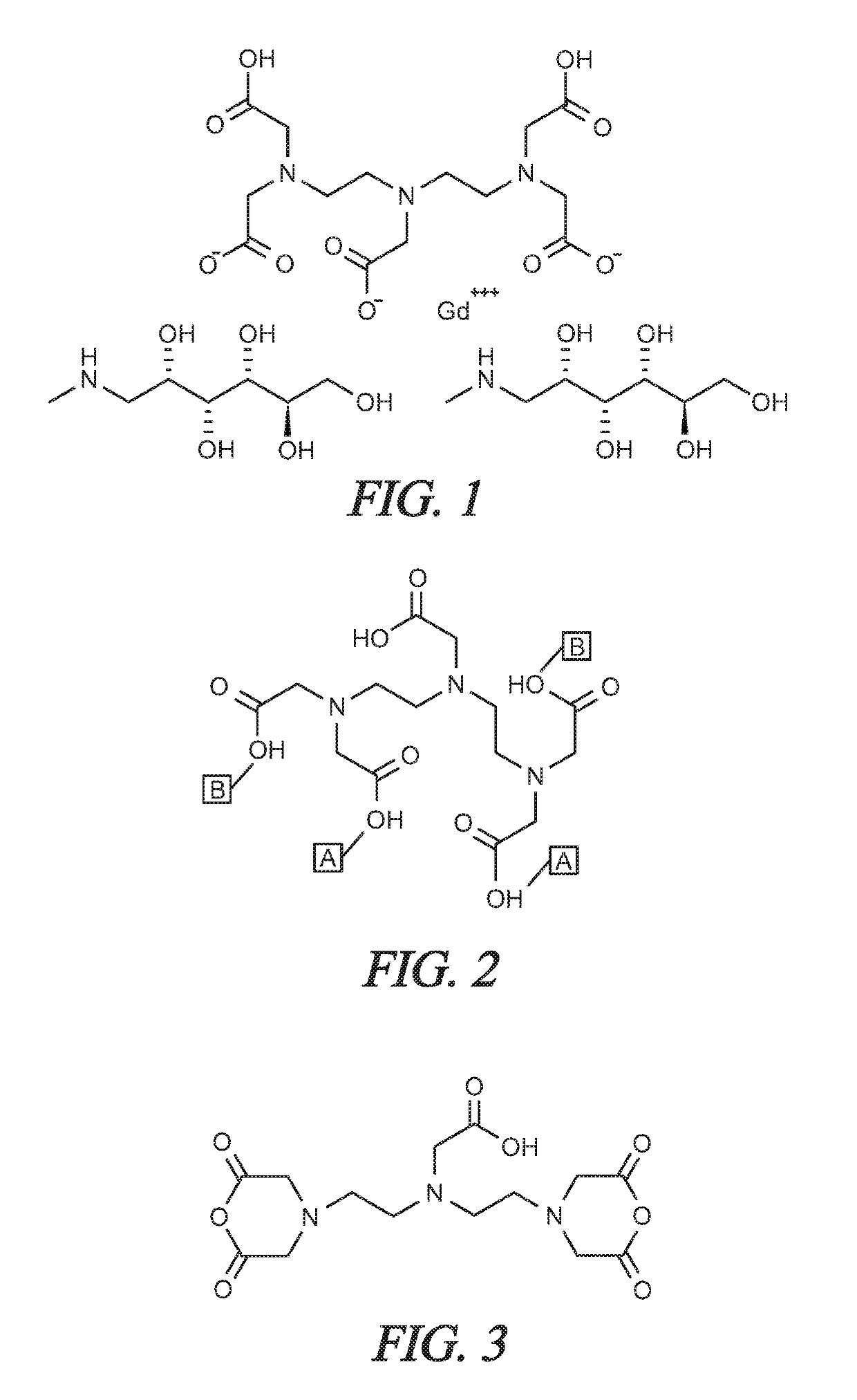
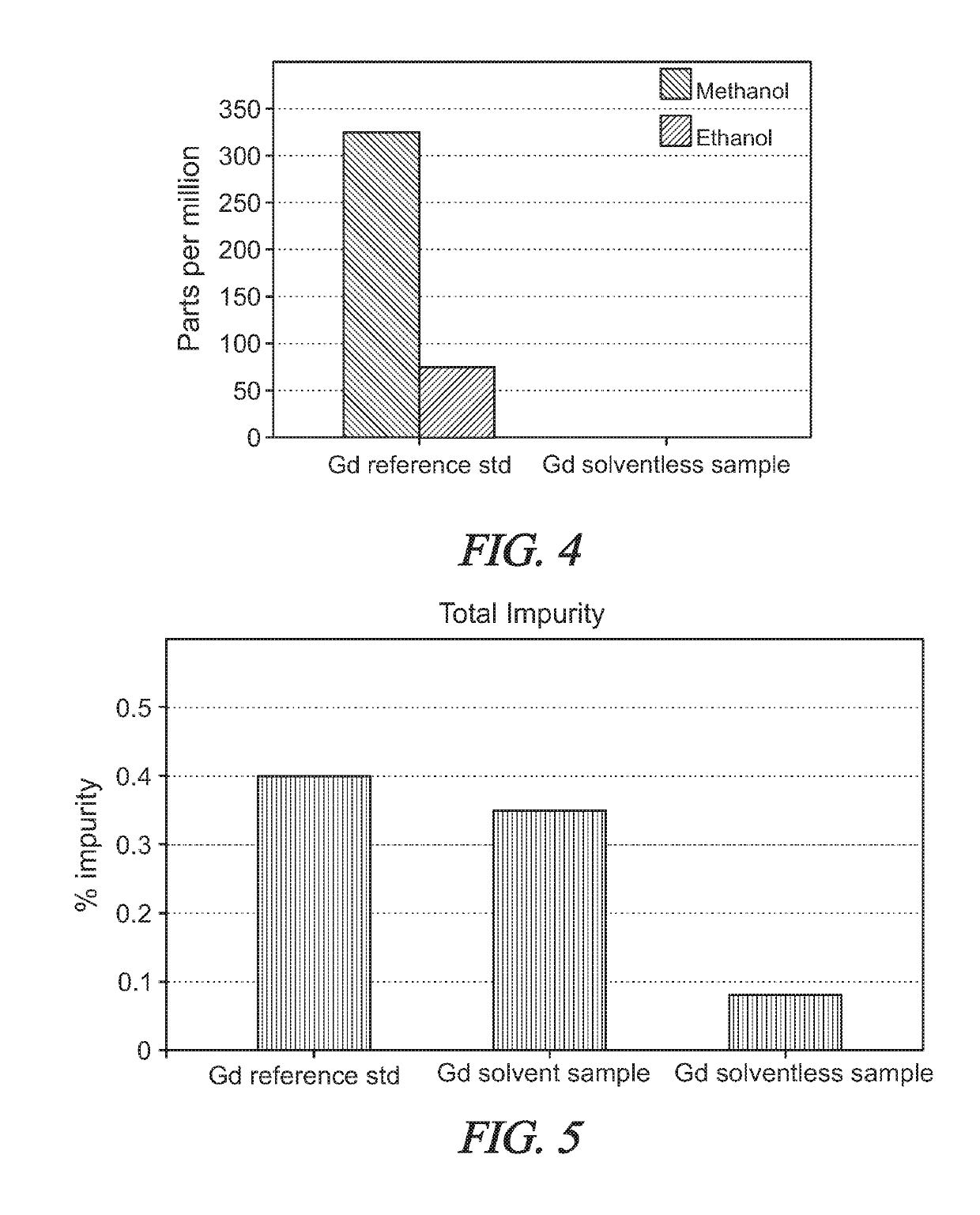
Solvent-free gadolinium contrast agents
a gadolinium contrast agent, solvent-free technology, applied in the direction of medical preparations, emulsion delivery, dispersed delivery, etc., can solve the problems of insufficient resolution of toxicity problems, inability to use gadolinium metal in vivo, inability to completely solve toxicity problems, etc., to reduce the amount of intentional excess pentetic acid, reduce the variability of parameters, and cause skin irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ree Gadopentetate Dimeglumine
Raw Materials
[0176]
Raw MaterialMole Ratio1DTPA12Gd2O30.4963Water2.2V1 (DTPA)4Meglumine0.992
Preparation of DTPA Solution
[0177]1. Heat reactor to 25-30° C.[0178]2. Charge water[0179]3. Begin stirring (stir unless otherwise indicated, nominal rate 300 rpm)[0180]4. Charge 10% of the DTPA[0181]5. Stir until uniformly distributed in the water[0182]6. If all the DTPA is charged, then go to step 8[0183]7. Go to step 4[0184]8. Stir 10 min
Preparation of Gadolinium: DTPA Complex
[0185]9. Charge 25% by weight of the Gadolinium oxide[0186]10. Stir until uniformly distributed[0187]11. If all the Gadolinium oxide is charged then go to step 13[0188]12. Go to step 9[0189]13. Stir 10 min[0190]14. Raise temperature to 95+ / −2° C.[0191]15. Stir 3 hrs.[0192]16. Check clarity[0193]17. If not clear continue for 1 hr, go to step 16[0194]18. If clear, continue 1 hr and then cool to 40-45° C.[0195]19. If precipitate forms, heat to 95+ / −2° C. and stir for 1 hr, go to step 16
Verify C...
example 2
ree Gadoterate Meglumine
[0238]Preparation of DOTA Solution[0239]1. Heat reactor to 25-30° C.[0240]2. Charge water[0241]3. Begin stirring (stir unless otherwise indicated, nominal rate 300 rpm)[0242]4. Charge 10% of the DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid)[0243]5. Stir until uniformly distributed in the water[0244]6. If all the DOTA is charged, then go to step 8[0245]7. Go to step 4[0246]8. Stir 10 min
[0247]Preparation of Gadolinium: DOTA Complex[0248]9. Charge 25% by weight of the Gadolinium oxide[0249]10. Stir until uniformly distributed[0250]11. If all the Gadolinium oxide is charged then go to step 13[0251]12. Go to step 9[0252]13. Stir 10 min[0253]14. Raise temperature to 95+ / −2° C.[0254]15. Stir 3 hrs.[0255]16. Check clarity[0256]17. If not clear continue for 1 hr, go to step 16 (this step took about 12 hours, slower than Magnevist synthesis)[0257]18. If clear, continue 1 hr and then cool to 40-45° C.[0258]19. If precipitate forms, heat to 95+ / −2° C. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com