Methods and Compositions for RNAi Mediated Inhibition of Gene Expression in Mammals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment a
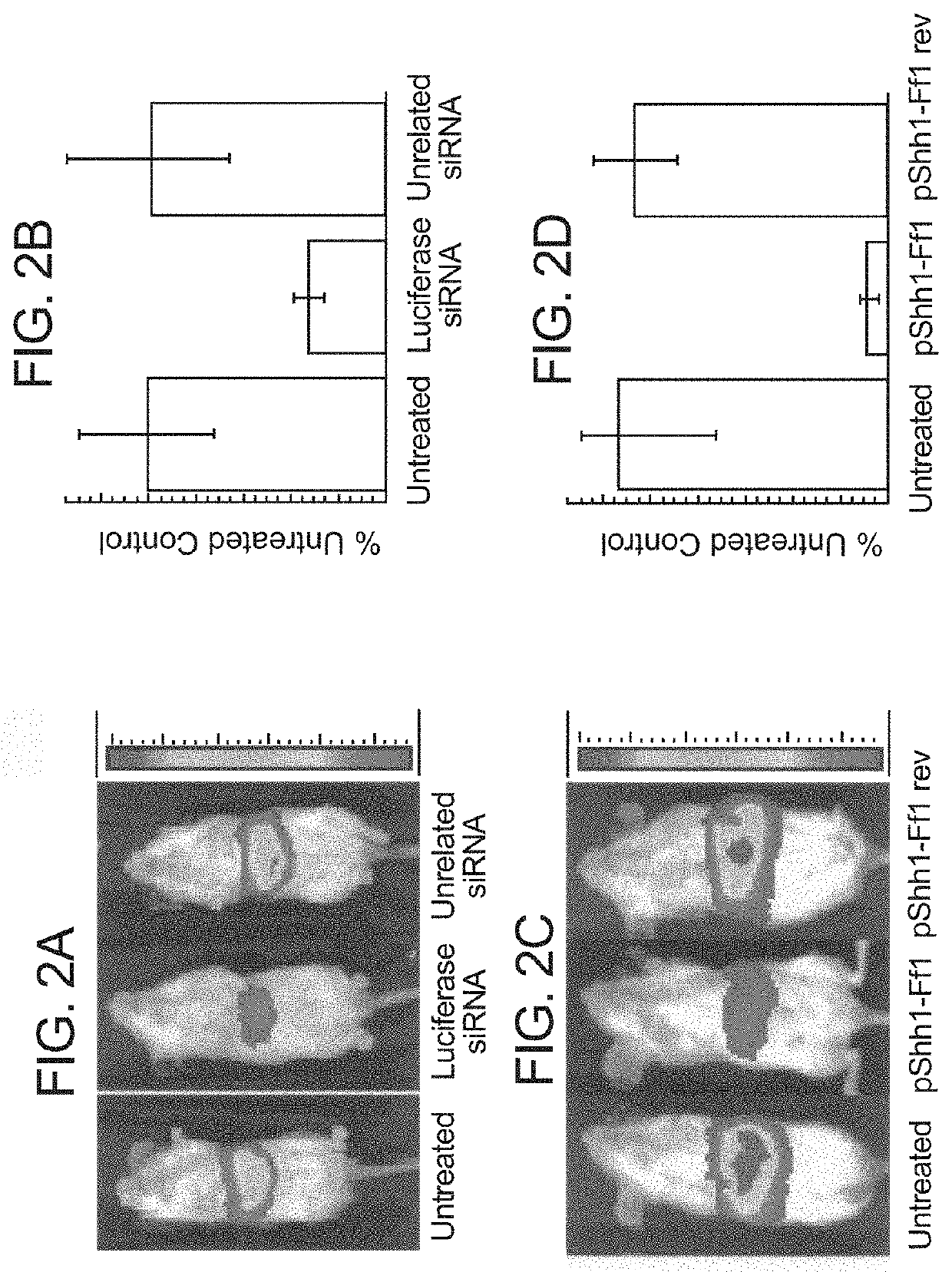
[0178]control group: RNAs containing the HCV IRES and a luciferase reporter sequence are injected into mice and they glow when this RNA is translated into luciferase protein
[0179]Coinject inhibitor with RNA. Both go to the same cells. Inhibition is expressed as activity (glowing) compared to control group.
experiment b
[0180]Same as experiment A except we inject a DNA that encodes the target RNA along with the inhibitor. The DNA goes to the nucleus of the mouse hepatocytes and is transcribed to give the target RNA. This RNA goes to the cytoplasm of the cells where it interacts with the inhibitor.
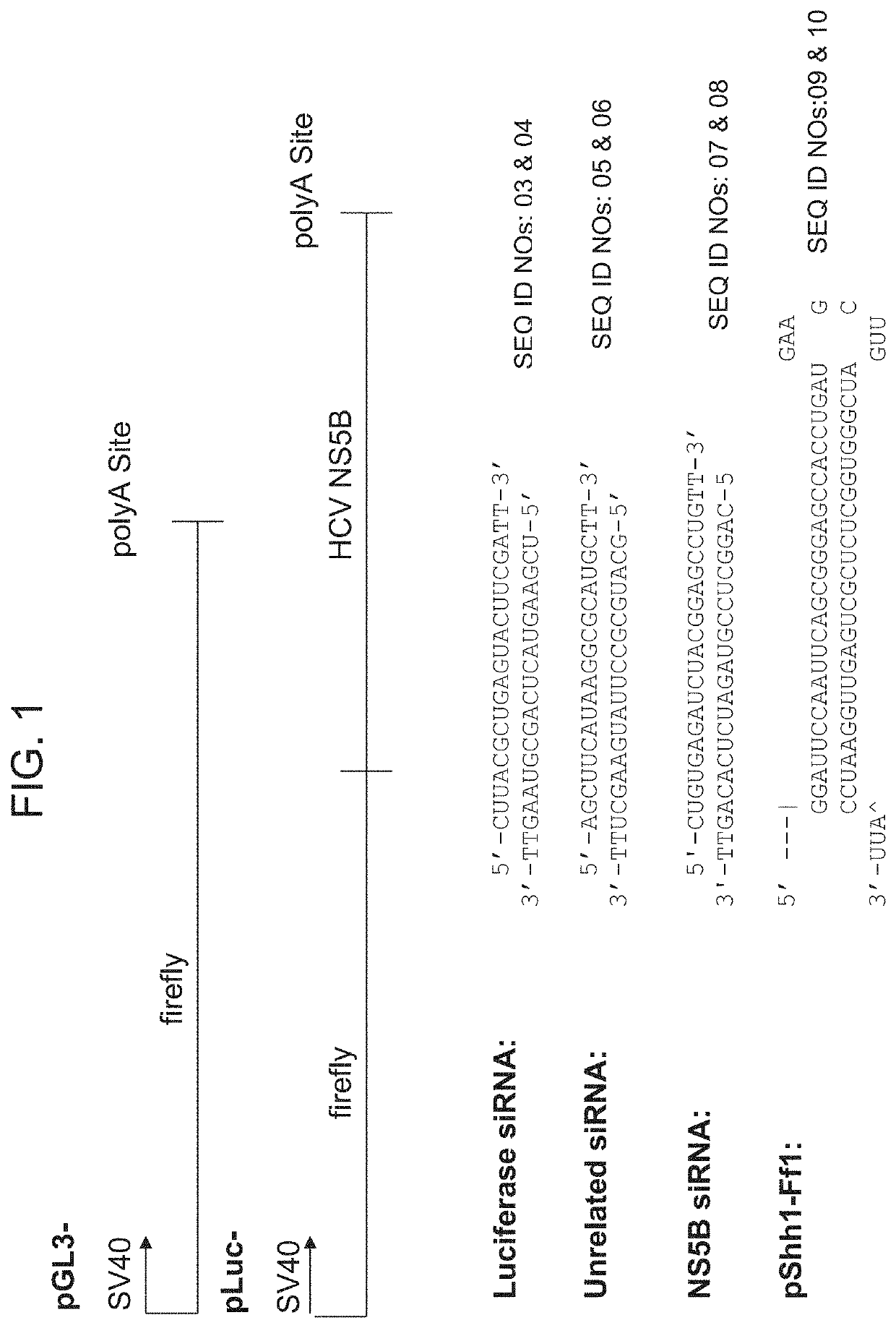
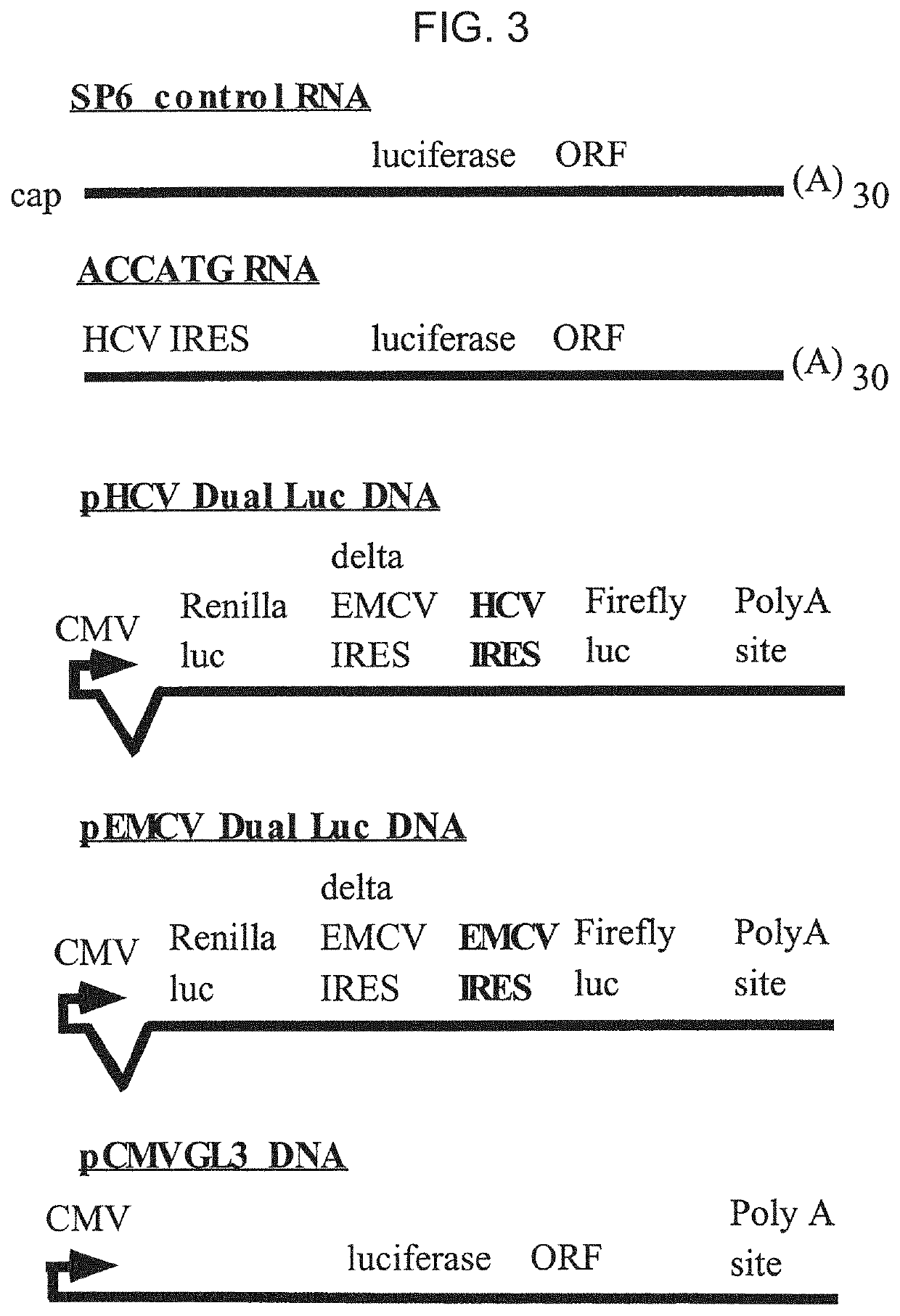
[0181]The constructs employed in these experiments are provided in FIG. 4.
The results of these experiments with antisense and DNAzyme inhibitors are provided in FIGS. 5A to 5F.
III. Inhibition of Hepatitis B Virus Replication in Mice by RNA Interference
A. Methods
1. Plasmids
[0182]pTHBV2 (as described in Marion et al., In Frontiers in Viral Hepatitis. (ed. R. F. Schinazi, C. R., and J-P. Sommadossi) 197-209 (Elsevier Science, Amsterdam, 2002)) contains the HBV genome plus a redundancy for the sequences between nucleotides 1067 and 1996 of the HBV genome. HBVU6 RNAi plasmids were cloned using methods described at the website produced by placing http: / / before and “cshl.org:9331 / RNAi / docs / Web_version_of_PCR_stra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gene expression profile | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com