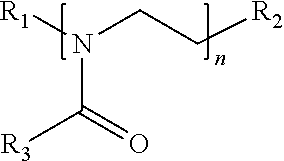
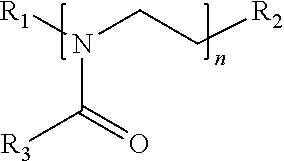
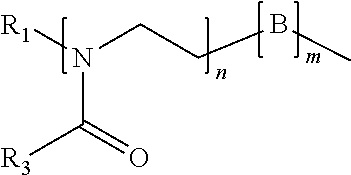
Surface disinfectant with residual biocidal property
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0135]The following examples illustrate liquid formulations made in accordance with aspects of the present invention. The testing results on these formulations demonstrate the desired residual sanitizing or disinfecting performance once being applied onto surfaces and dried. Cleaning performance is also tested on those formulations that not only provide residual disinfecting benefit but also cleaning features.
[0136]Formulations were tested for residual efficacy using the EPA 01-1A protocol. Briefly, bacteria were added to a glass slide and allowed to dry on the surface. The formulation was then sprayed onto the surface and dried to form a transparent film. Once a film had formed, the glass slide was exposed to alternating wet and dry cycles using the Gardner wear tester as described in the protocol. In between each cycle the slide was re-inoculated with bacteria. After the appropriate number of wear and re-inoculations (48 passes and 11 re-inoculations for healthcare formulation and...
example — wipe
Example—Wipe formulation
[0155]Wipe formulations A-I were prepared and tested on various substrates.
[0156]In the tests for Formulations A-F, 8 grams of formulation was added to a 7″×8″, 100% PP, 34 gram per square meter substrate. The substrate, which was obtained from Rockline Industries, was a Kimberly Clark melt blown product. The results are shown in Table 12 below.
TABLE 12ABCDEF(wt %)(wt %)(wt %)(wt %)(wt %)(wt %)Aquazol 5001.501.501.500.600.901.20BTC 8850.450.901.431.001.001.00(50% Active)Tomadol 25-120.200.200.200.200.200.20Fragrance0.100.100.100.100.100.10WaterbalancebalancebalancebalancebalancebalanceLog2.4 3.9 4.7 2.8 3.2 Reduction*Result*FailPassPassFailFailPass*Clorox method 01-1A results - The method was adapted to wipes by folding the substrate lengthwise into 1″ folds then wrapping the folded substrate strip across the second, third and fourth fingers. One pass was made across the glass carriers. A log reduction greater than 3.0 was required to be considered passing.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com