Multi-Day Patch for the Transdermal Administration of Rotigotine
a transdermal therapy and patch technology, applied in the direction of muscular disorders, nervous disorders, drug compositions, etc., can solve the problems of inability to prepare limited capacity of solvent-based transdermal therapeutic systems known from the prior art, etc., to achieve the effect of reducing the concentration of rotigotine and being prepared quickly and cost-effectively
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
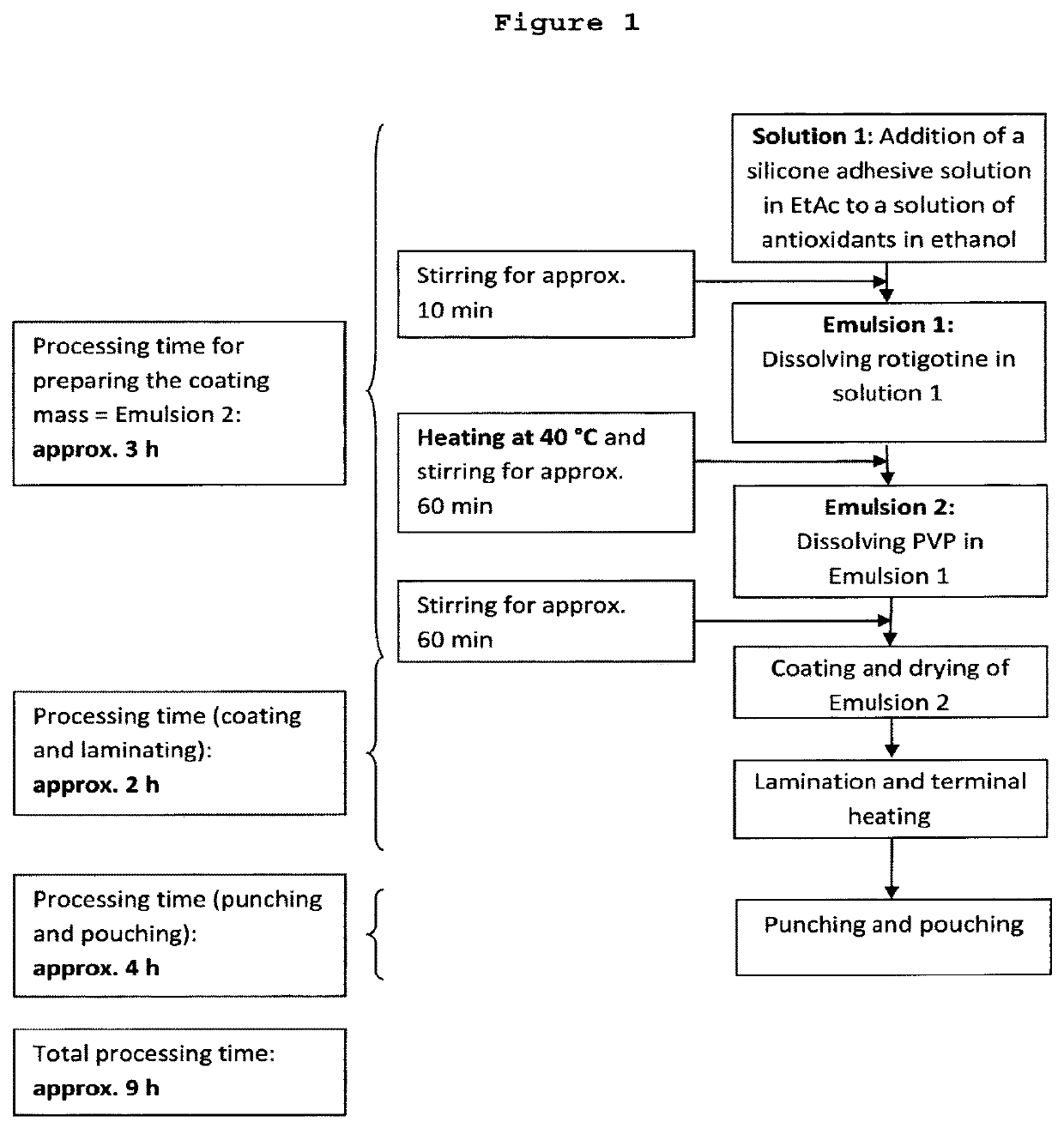
[0219]4-Day mono-layer TTS comprising a reservoir layer having a coating weight of 150 g / m2 and containing 9 wt.-% rotigotine and 2 wt.-% PVP; solvent system used for the preparation method: heptane / ethanol (1.4:1 (w / w)) 18.44 kg silicone adhesive 7-4301 (73 wt.-% in heptane) were mixed with the following components under permanent stirring until a homogeneous dispersion was obtained:[0220]1. 2.44 kg of an ethanolic solution containing 25 wt.-% polyvinylpyrrolidone (KOLLIDON® F. 90), 0.11 wt.-% aqueous sodium metabisulfite solution (10 wt.-%), 0.25 wt.-% ascorbyl palmitate and 0.62 wt.-% DL-alpha-tocopherol;[0221]2. 9.131 kg of an ethanolic solution containing 2.724 kg rotigotine obtained by dissolving rotigotine of polymorphic Form I;[0222]3. 18.43 kg of silicone adhesive 7-4201 (73 wt.-% in heptane); and[0223]4. 1.579 kg heptane.
[0224]For the manufacture of the self-adhesive matrix layer, the obtained dispersion was coated onto a suitable release liner (e.g. SCOTCHPAK™ 9744) and t...
example 2
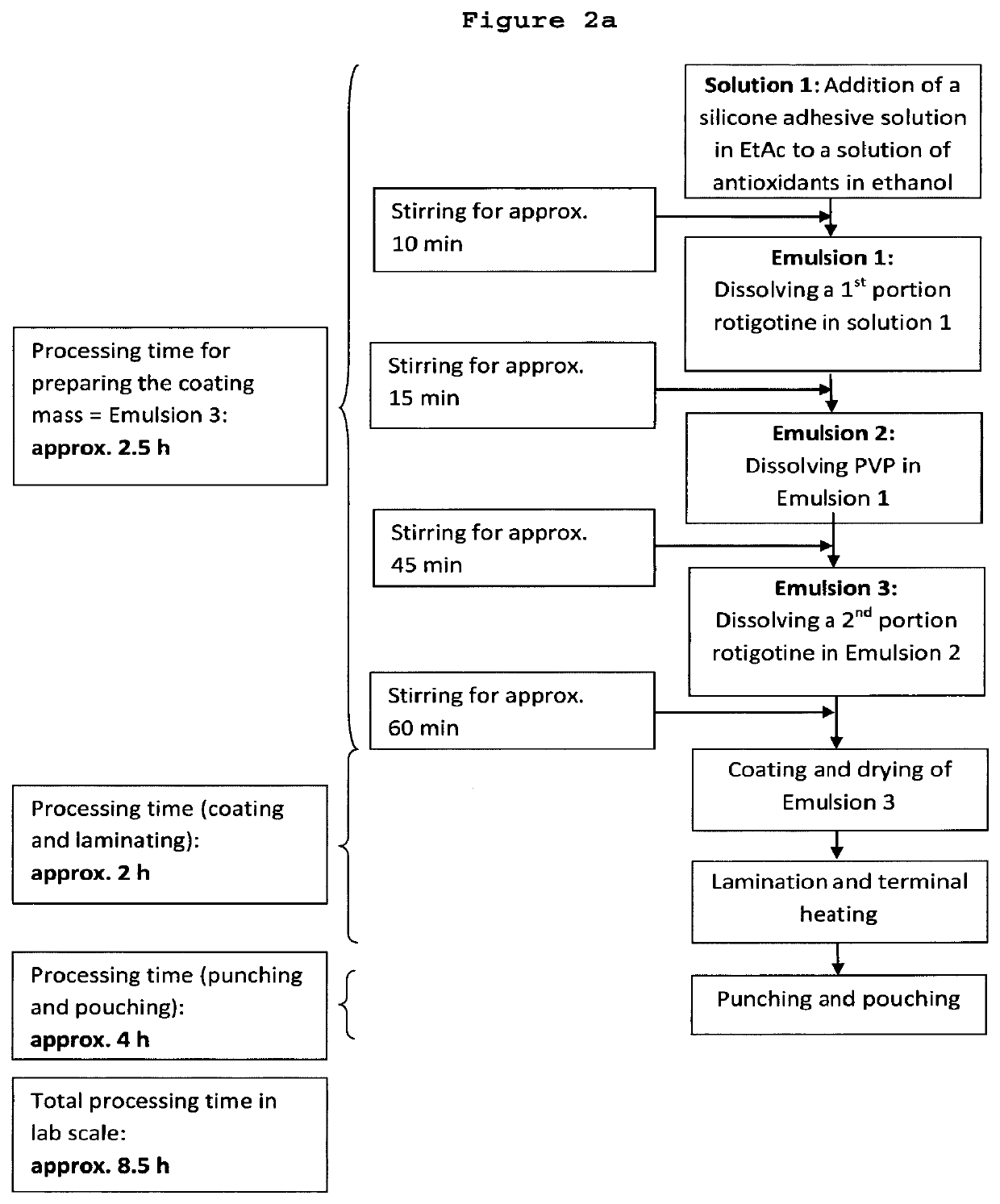
[0226]7-Day bi-layer TTS comprising (a) a reservoir layer having a coating weight of 150 g / m2 and containing 18 wt.-% rotigotine and 4 wt.-% PVP and (b) a skin adhesive layer containing no rotigotine and having a coating weight of 18 g / m2; solvent system used for the preparation method: heptane / ethanol (1.4:1 (w / w))
Preparation of the Reservoir Layer Matrix (Step 1)
[0227]9.66 kg silicone adhesive 7-4301 (73 wt.-% in heptane) were mixed with the following components under permanent stirring until a homogeneous dispersion was obtained:[0228]1. 2.90 kg of an ethanolic solution containing 25 wt.-% polyvinylpyrrolidone (KOLLIDON F 90), 0.11 wt.-% aqueous sodium metabisulfite solution (10 wt.-%), 0.25 wt.-% ascorbyl palmitate and 0.62 wt.-% DL-alpha-tocopherol;[0229]2. 6.98 kg of an ethanolic solution containing 3.26 kg rotigotine obtained by dissolving rotigotine of polymorphic Form I;[0230]3. 9.66 kg of silicone adhesive 7-4201 (73 wt.-% in heptane); and[0231]4. 0.82 kg heptane.
Preparati...
example 3
[0255]7-Day bi-layer TTS comprising (a) a reservoir layer having a coating weight of 150 g / m2 and containing 18 wt.-% rotigotine and 8 wt.-% PVP and (b) a skin adhesive layer containing no rotigotine and having a coating weight of 18 g / m2; solvent system used for the preparation method: ethyl acetate / ethanol (5:1 (w / w))
Preparation of the Reservoir Layer Matrix (Step 1)
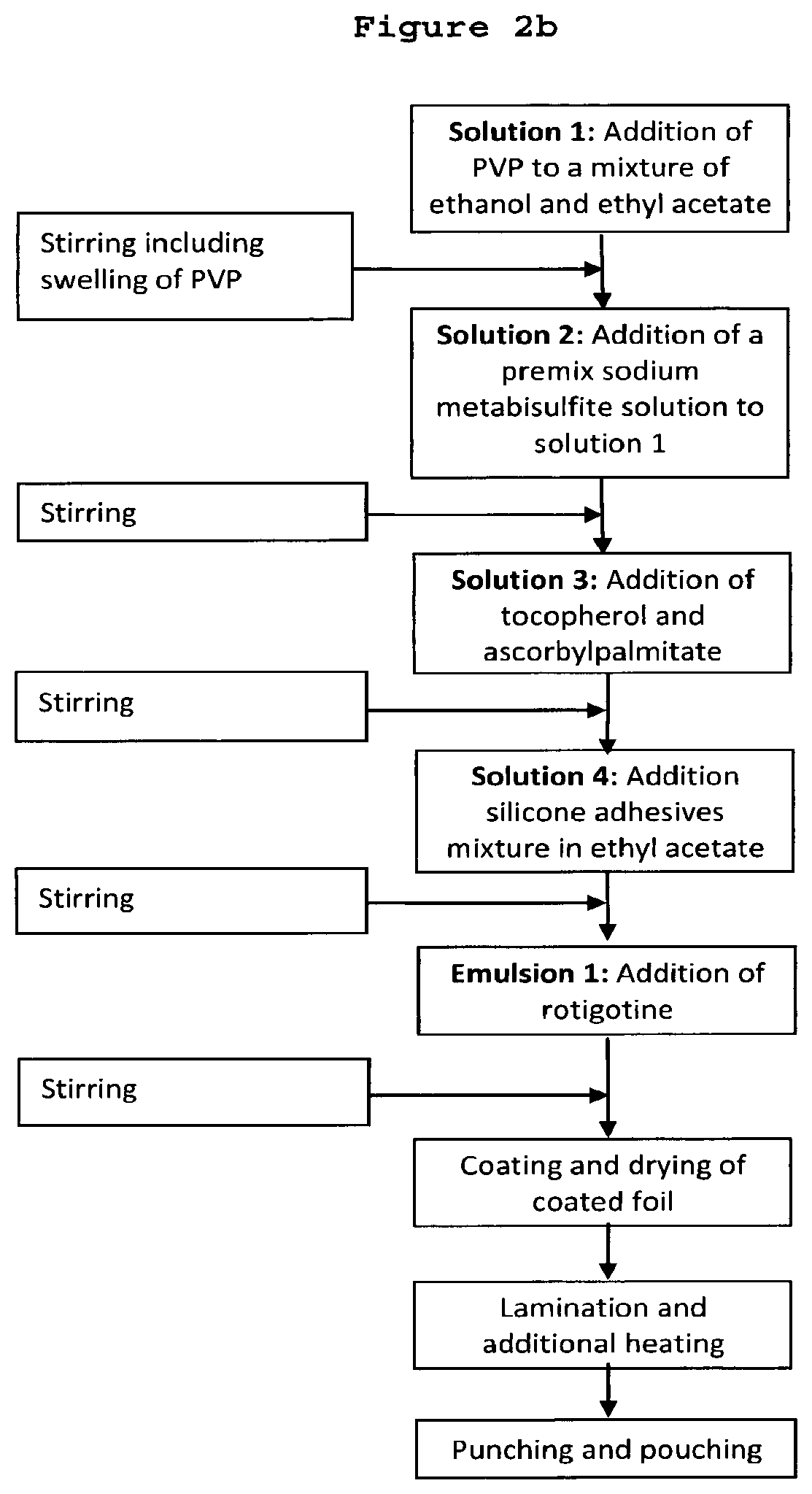
[0256]0.061 g DL-a-Tocopherol, 0.024 g ascorbyl palmitate and 0.020 g of an aqueous sodium metabisulfite solution (10 wt.-%) were mixed with 6.0 g anhydrous ethanol to obtain a clear solution.
[0257]38.0 g silicone adhesive 7-4202 (59.1 wt.-% in ethyl acetate) and 36.9 g silicone adhesive 7-4302 (60.9 wt.-% in ethyl acetate) were added to the obtained solution of antioxidants and stirred at 400 rpm. After approximately 10 min, 11.0 g rotigotine of polymorphic Form II were added while stirring. The mixture was heated up to 40° C. and stirred at 400 rpm until a homogenous dispersion was obtained. Thereafter 4.9 g polyviny...
PUM
| Property | Measurement | Unit |
|---|---|---|
| processing time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| dynamic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com