Hyaluronic acid derivative and use thereof
a technology of hyaluronic acid and derivatives, which is applied in the field of hydrogels, can solve the problems of reducing the amount of hyaluronic acid, collagen, elastin, and other matrix polymers present in the skin, affecting the effect of skin elasticity, and affecting the appearance of skin,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation examples
Preparation Example 1
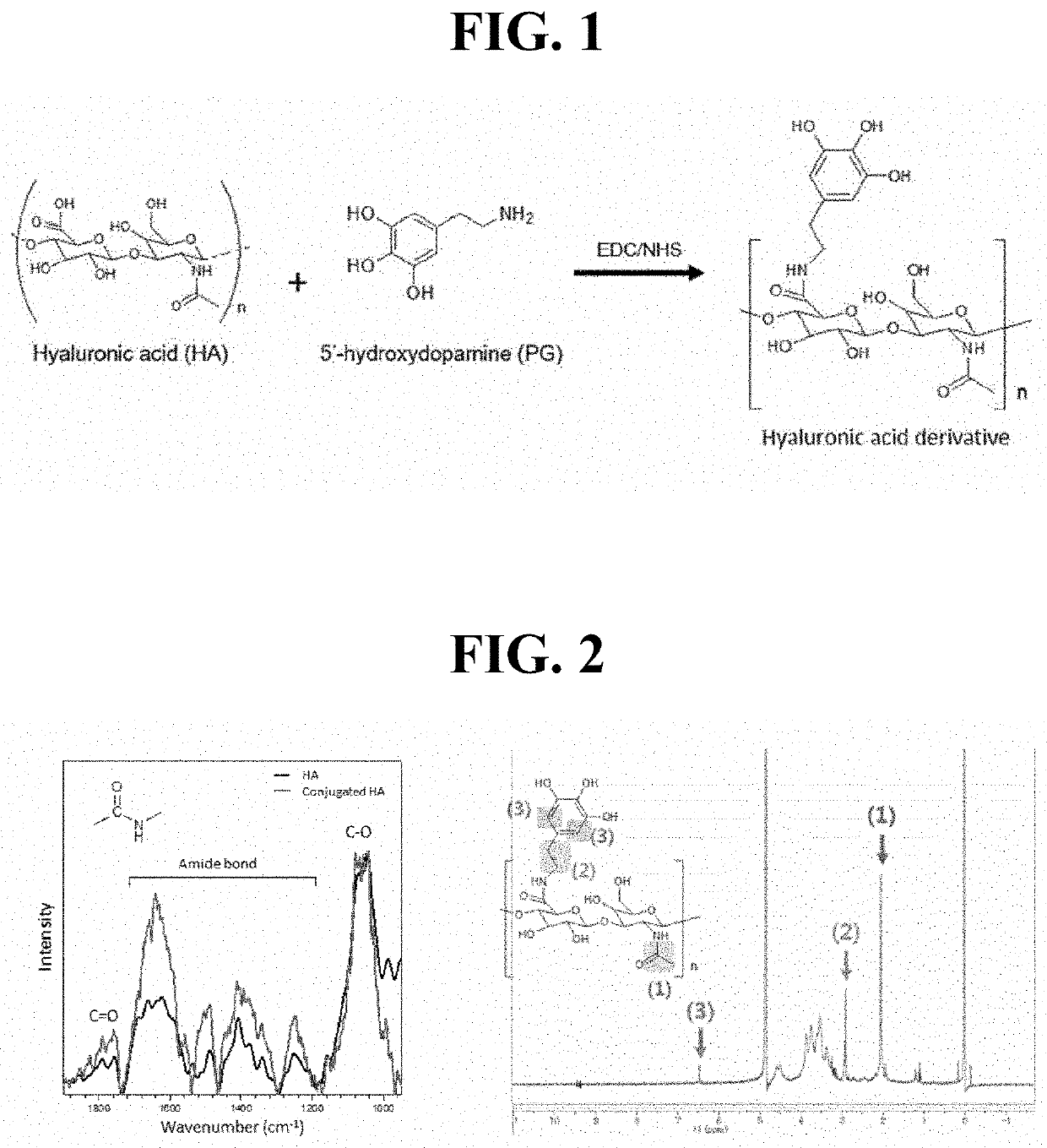
[0104]Preparation of Hyaluronic Acid Derivative Modified with Gallol Group
[0105]As illustrated in FIG. 1, a hyaluronic acid derivative modified with a gallol group according to the present invention was prepared. Specifically, hyaluronic acid (MW 200K, Lifecore Biomedical, LLC, IL, USA) was completely dissolved in water (TDW), and 1 equivalent of N-hydroxysuccinimide (NHS, Sigma-Aldrich, Inc., St. Louis, Mo., USA) and 1.5 equivalent of 1-(3-dimethylaminopropyl)-3-ehtylcarbodiimide hydrochloride (EDC, Thermo Scientific, Rockford, Ill., USA) were added thereto. After 30 minutes, 1 equivalent of 5′-hydroxydopamine (Sigma-Aldrich, Inc.) was added thereto as a PG moiety and the resultant was allowed to react at pH 4 to 4.5 for 24 hours. Reactants having two different molar ratios were used in the preparation of HA-PG conjugates (HA:EDC:NHS:PG=1:1.5:1:1 or 2:1.5:1:1). Then, EDC, NHS, and 5′-hydroxydopamine were removed by dialysis based on PBS and water (Cellu / Sep (tr...
preparation example 2
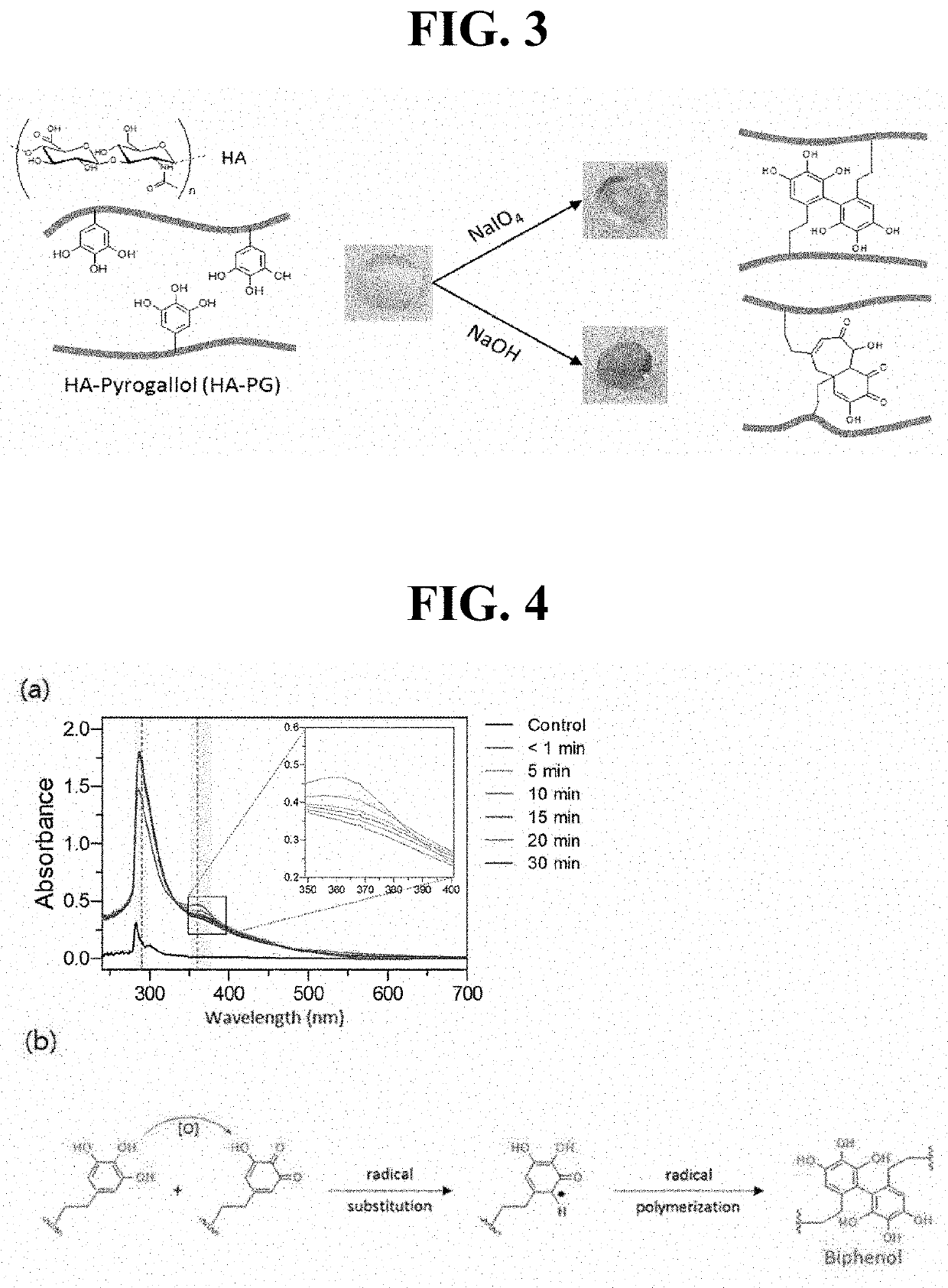
[0106]Preparation of Hyaluronic Acid Hydrogel
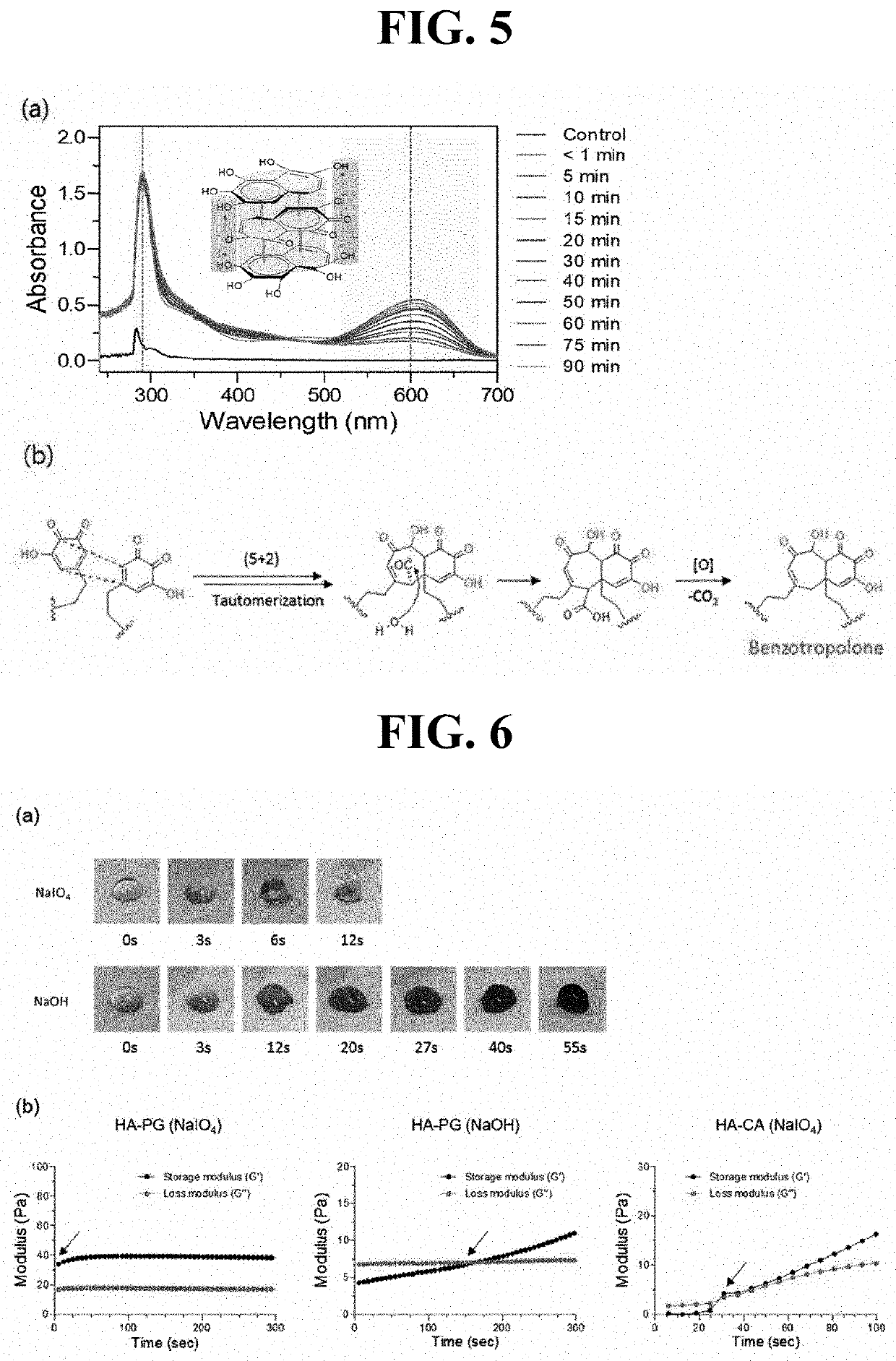
[0107]The hyaluronic acid derivatives of Preparation Example 1 were crosslinked to prepare a hyaluronic acid hydrogel, in which each crosslinking method using sodium periodate (NaIO4) as an oxidizing agent or sodium hydroxide (NaOH) as a pH adjusting agent (pH 8 to 9) was employed. Specifically, the hyaluronic acid derivatives were dissolved in PBS (1% (w / v), 2% (w / v)), and then crosslinking was allowed to proceed while performing mixing with 4.5 mg / ml of NaIO4 or 0.08 M NaOH at a volumetric ratio of 1.5:1 to 4:1 relative to the hyaluronic acid derivative solution. As illustrated in FIG. 3, through each of these crosslinking methods, a light brown-colored hyaluronic acid hydrogel or a blue-colored hyaluronic acid hydrogel was prepared.
[0108]In order to specifically identify crosslinking of the hyaluronic acid hydrogel, analysis with ultraviolet-visible spectroscopy (UV-vis) was performed. In a case of using NaIO4, as illustrated in FIG. 4...
example 1
[0109]Changes in Physical Properties of Hyaluronic Acid Hydrogel Depending on Crosslinking Method
[0110]In the present example, changes in physical properties of hyaluronic acid hydrogels, depending on difference in crosslinking method in Preparation Example 2, were compared. On the other hand, in the hydrogel preparation step, a molar concentration ratio of hyaluronic acid to 5′-hydroxydopamine (0.5×(HA:EDC:NHS:5′-hydroxydopamine=2:1.5:1:1), lx (HA:EDC:NHS:5′-hydroxydopamine=1:1.5:1:1)) could be used to adjust a rate of being substituted with a gallol group to 4% to 5% (0.5×) or 8% to 9% (1×) (not shown), and changes in physical properties of hydrogels depending on a degree of substitution of 5′-hydroxydopamine were also compared. Specifically, hydrogel formation and changes in elastic modulus over time were compared depending on crosslinking methods; and elasticity, adhesive strength, swelling, and degradation patterns depending on the crosslinking method and the degree of substitu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com