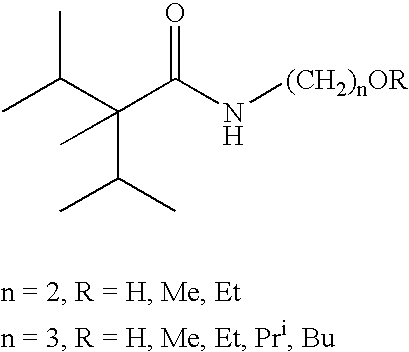Compounds with physiological cooling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1 preparation
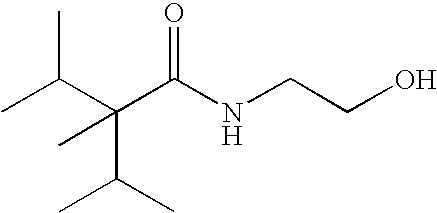
of N-(2-hydroxyethyl)-2,3-dimethyl-2-isopropylbutyramide (1)
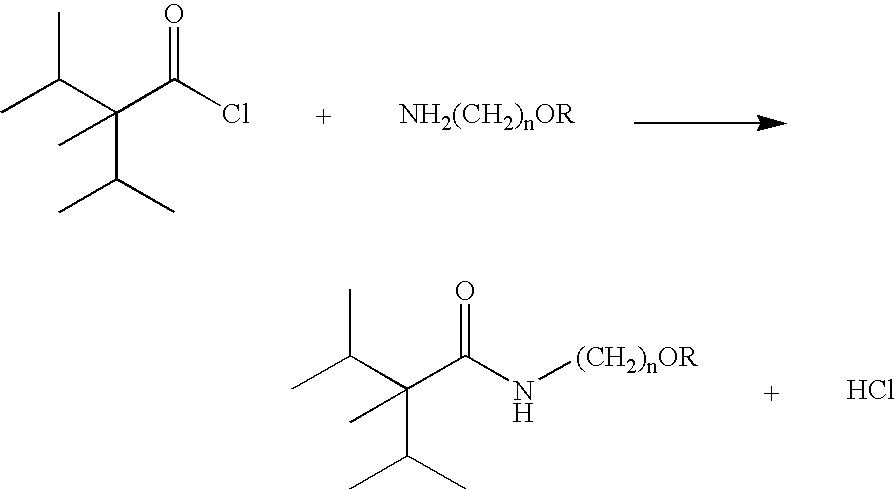
[0019]A mixture of 19.5 g 2-aminoethanol and 50 mL anhydrous hexanes was cooled to 0° C. Under mechanical stirring 20 g freshly distilled 2,3-dimethyl-2-isopropylbutyryl chloride was added dropwise over a period of one hour while maintaining the reaction temperature below 5° C. The reaction mixture was then stirred for one more hour at this temperature followed by one hour at room temperature.
[0020]50 mL water was added and the top organic layer was separated and washed with water till is neutral. The solvent was evaporated and 21.5 g crude product (99+% GC purity) was obtained. The product was recrystallized from acetone / water.
example 2 preparation
of N-(3-hydroxypropyl)-2,3-dimethyl-2-isopropylbutyramide (4)
[0021]The procedure of Example 1 was repeated using 3-aminopropyl in place of 2-aminoethanol. N-(3-hydroxypropyl)-2,3-diemthyl-2-isopropylbutyramide (4, 99+% GC purity) was obtained as very viscous oil.
example 3 preparation
of N-(2-methoxyethyl)-2,3-dimethyl-2-isopropylbutyramide (2)
[0022]A mixture of 7.74 g 2-methoxyethylamine, 10.95 g triethylamine and 50 mL anhydrous hexanes was cooled to below 5° C. and under mechanical agitation 20 g freshly distilled 2,3-dimethyl-2-isopropylbutyryl chloride was added dropwise over a period of 30 minutes while maintaining the reaction temperature below 25 ° C. After addition the reaction mixture was stirred for 30 minutes at this temperature followed by one hour at room temperature.
[0023]50 mL water was added, the top organic layer was separated and washed with 20 mL 5% NaOH solution followed by water till it was neutral. The solvent was evaporated and 24.2 g crude product (2. 98.5+% GC purity) was obtained as viscous oil.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com