Detecting lipocalin
a technology of lipocalin and lipocalin, which is applied in the field of detection of lipocalin, can solve the problems of medical practitioners and inability to find them
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
HNL Loop Regions as Antigens for Producing Antibodies
[0097]The amino acid sequence of human neutrophil gelatinase-associated lipocalin is as follows (SEQ ID NO:1).
[0098]
1QDSTSDLIPAPPLSKVPLQQNFQDNQFQGKWYVVGLAGNA41ILREDKDPQKMYATIYELKEDKSYNVTSVLFRKKKCDYWI81RTFVPGCQPGEFTLGNIKSYPGLTSYLVRVVSTNYNQHAM121VFFKKVSQNREYFKITLYGRTKELTSELKENFIRFSKSLG161LPENHIVFPVPIDQCID
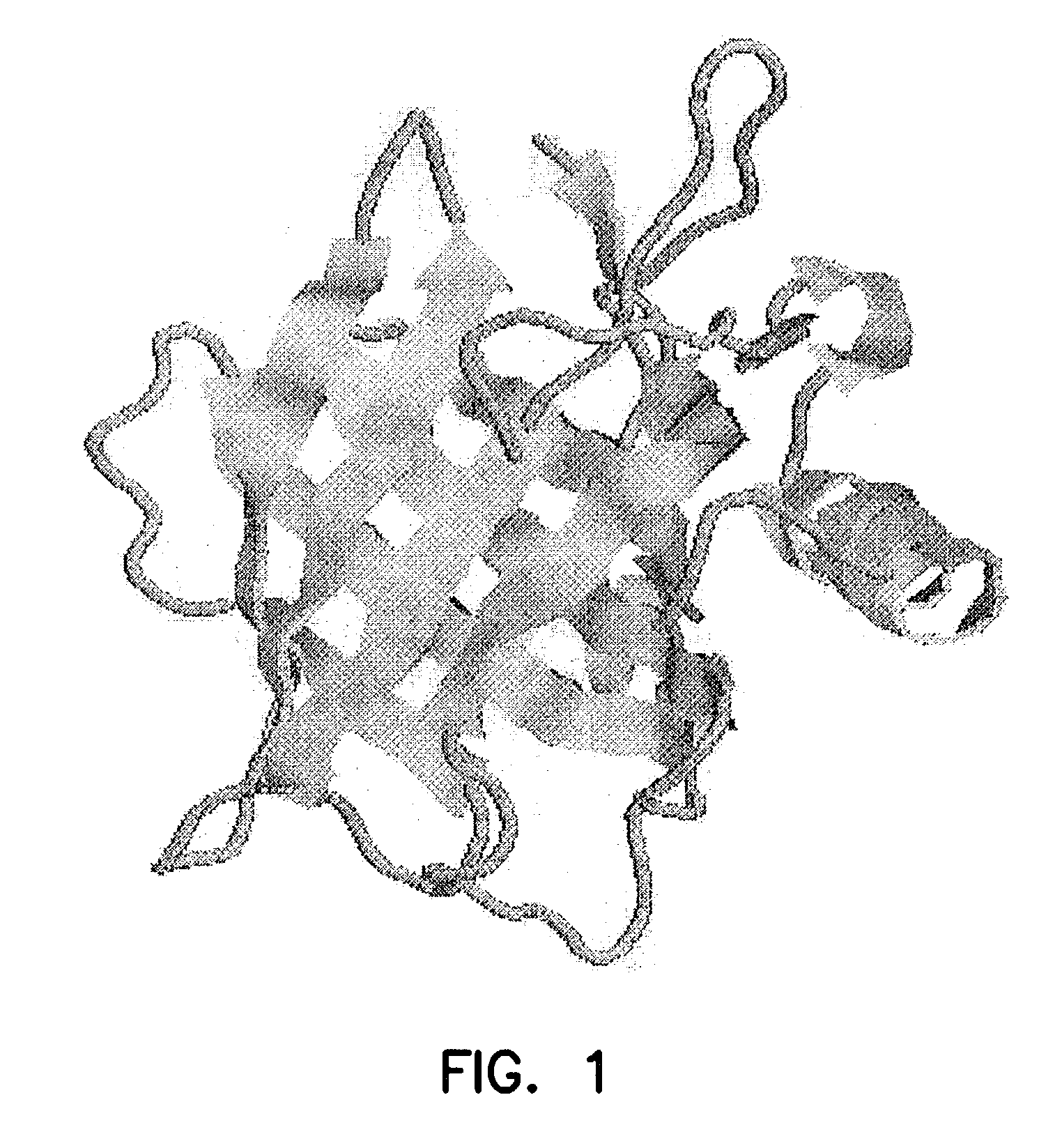
[0099]Lipocalin contains eight surface-accessible loops. These areas are shown as thin yellow bands in FIG. 1. Two of these loops flank the central lipocalin beta barrel. The loop areas make good epitope targets because they are solvent exposed. The sequences of the loop regions are as follows:
[0100]
1.Pro17 to Gln28:PLQQNFQDNQFQ(SEQ ID NO: 2)2.Asn39 to Tyr52:NAILREDKDPQKMY(SEQ ID NO: 3)3.Lys59 to Ser63:KEDK(SEQ ID NO: 4)4.Arg72 to Lys75:RKKK(SEQ ID NO: 5)5.Gly86 to Gly90:GCQPG(SEQ ID NO: 6)6.Gly95 to Gly102:GNIKSYPG(SEQ ID NO: 7)7.Asn114 to Gln117:NYNQ(SEQ ID NO: 8)8.Phe168 to Asp177:FPVPIDQCID(SEQ ID NO: 9)
[0101]Any peptide within ...
example 2
Antibody Generation
[0105]This Example illustrates that specific lipocalin peptides can be used to generate antibodies that are reactive with lipocalin.
Peptides
[0106]Peptides were chosen based upon the loop regions of human neutrophil lipocalin described in the X-ray crystallographic structure published by Goetz et al. Biochemistry 2000, 39, 1935–1941. Specific peptides were chosen for conjugation to KLH protein. These peptides had the following sequences:
[0107]
SEQ ID NO: 12 - YELKEDKSSEQ ID NO: 13 - LSKVPLQQNFQDNQSEQ ID NO: 14 - GNAILREDKDPQKMYSEQ ID NO: 9 - FPVPIDQCID
[0108]KLH-conjugated peptides were synthesized and purified by HPLC by SigmaGenesis. Specifically, peptides having SEQ ID NO:12, 13 and 14 were synthesized with an N-terminal cysteine residue such that the peptide could be conjugated to KLH using succinimidyl m-maleimidobenzoate (MBS) as a coupling reagent. The peptide having SEQ ID NO:9 (FPVPIDQCID) was conjugated to KLH using activated EDC (1-ethyl-3(3-dimethylamino...
example 3
Hybridoma Cell Lines that Produce Monoclonal Antibodies
[0116]This Example illustrates that monoclonal antibodies can readily be prepared that are reactive with selected lipocalin epitopes.
Methods
[0117]Methods available in the art were utilized for in vitro and in vivo manipulation of monoclonal antibodies. Briefly, hybridoma cell lines expressing monoclonal antibodies were made by fusing B-cells from an in vivo-immunized mouse spleen with mouse myeloma cells using polyethylene glycol. Hybridoma cells were plated in several 96-well plates and were kept in culture for two weeks. Cell lines were screened for monoclonal antibodies using the ELISA method described above.
Results
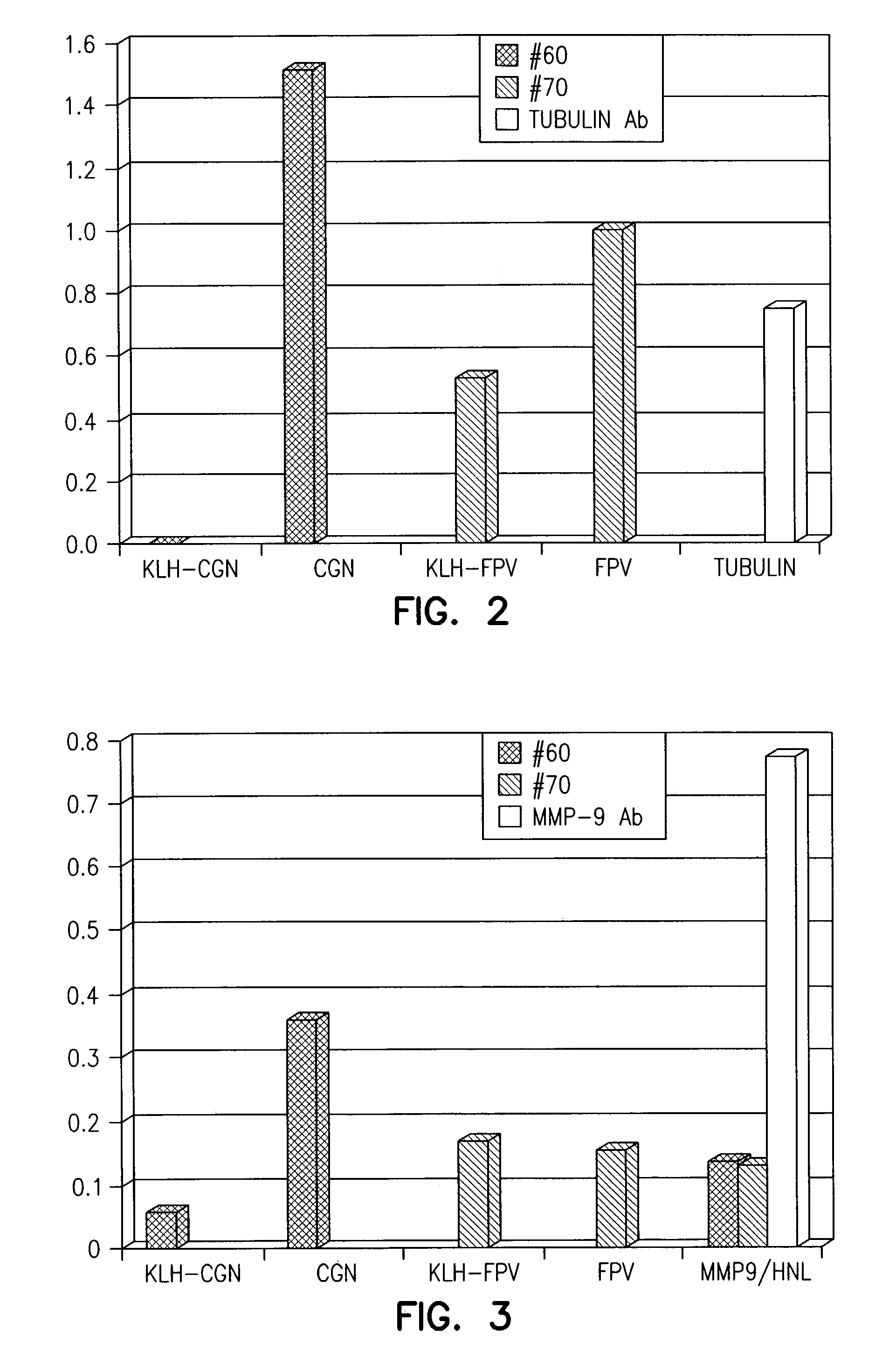
[0118]Supernatants (50 μL) were removed from each of 1032 wells containing hybridoma cells created from myeloma cells and B-cells from mouse #60 (immunized with KLH-SEQ ID NO:11). These supernatants were tested for monoclonal antibodies using the aforementioned ELISA test using plates coated with CGNAILREDKDPQKMY, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com