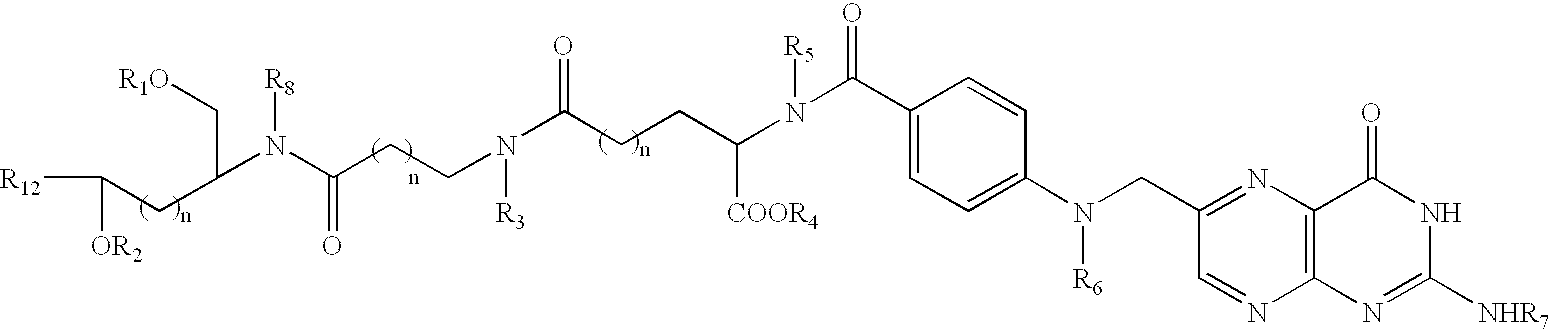
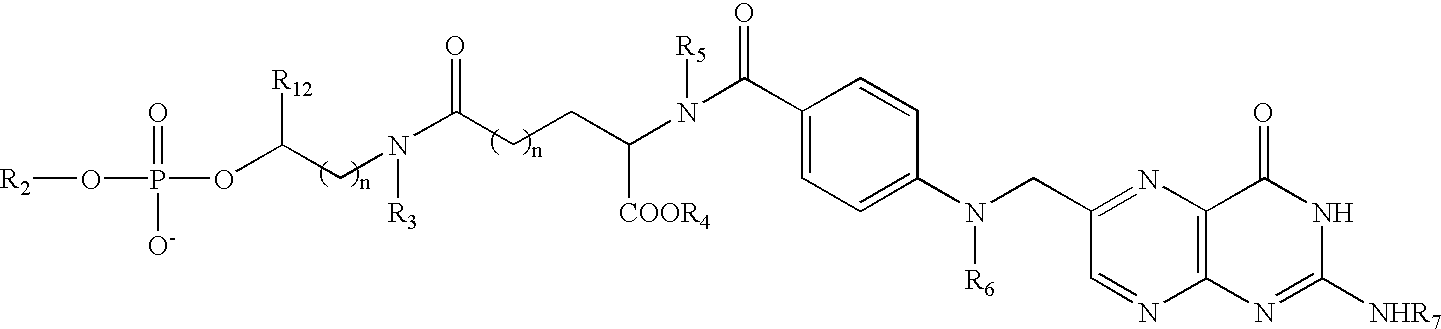
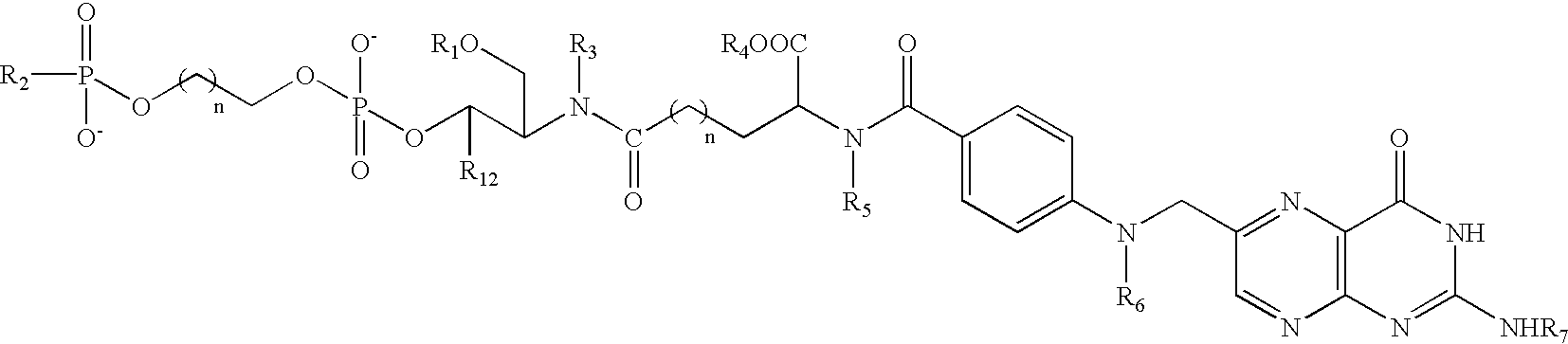
Conjugates and compositions for cellular delivery
a technology of conjugates and compositions, applied in the field of conjugates, compositions, methods of synthesis, can solve the problems of high toxicity to normal tissues, inability to efficiently conjugate, and inability to carry many compounds into living cells, etc., and achieves efficient coupling, efficient delivery and subsequent release, and easy conjugation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of O1-(4-Monomethoxytrityl)-N-(6-(N-(α-OFm-L-Glutamyl)Aminocaproyl))-D-Threoninol-N2-iBu-N10-TFA-Pteroic Acid Conjugate 3′-O-(2-Cyanoethyl-N,N-Diisopropylphosphor-Amidite) (20) (FIG. 5)
[0329]General. All reactions were carried out under a positive pressure of argon in anhydrous solvents. Commercially available reagents and anhydrous solvents were used without further purification. 1H (400.035 MHz) and 31P (161.947 MHz) NMR spectra were recorded in CDCl3, unless stated otherwise, and chemical shifts in ppm refer to TMS and H3PO4, respectively. Analytical thin-layer chromatography (TLC) was performed with Merck Art.5554 Kieselgel 60 F254 plates and flash column chromatography using Merck 0.040–0.063 mm silica gel 60.
[0330]N-(N-Fmoc-6-aminocaproyl)-D-threoninol (13). N-Fmoc-6-aminocaproic acid (10 g, 28.30 mmol) was dissolved in DMF (50 ml) and N-hydroxysuccinimide (3.26 g, 28.30 mmol) and 1,3-dicyclohexylcarbodiimide (5.84 g, 28.3 mmol) were added to the solution. The reacti...
example 2
Synthesis of 2-Dithiopyridyl Activated Folic Acid (30) (FIG. 9)
[0338]Synthesis of the cysteamine modified folate 30 is presented in FIG. 9. Monomethoxytrityl cysteamine 21 was prepared by selective tritylation of the thiol group of cysteamine with 4-methoxytrityl alcohol in trifluoroacetic acid. Peptide coupling of 21 with Fmoc-Glu-OtBu (Bachem Bioscience Inc., King of Prussia, Pa) in the presence of PyBOP yielded 22 in a high yield. N-Fmoc group was removed smoothly with piperidine to give 23. Condensation of 23 with p-(4-methoxytrityl)aminobenzoic acid, prepared by reaction of p-aminobenzoic acid with 4-methoxytrityl chloride in pyridine, afforded the fully protected conjugate 24. Selective cleavage of N-MMTr group with acetic acid afforded 25 in quantitative yield. Shiff base formation between 25 and N2-iBu-6-formylpterin 26,9 followed by reduction with borane-pyridine complex proceeded with a good yield to give fully protected cysteamine-folate adduct 27.12 The consecutive cleav...
example 3
Post Synthetic Conjugation of Enzymatic Nucleic Acid to Form Nucleic Acid-folate Conjugate (33) (FIG. 10)
[0339]Oligonucleotide synthesis, deprotection and purification was performed as described herein. 5′-Thiol-Modifier C6 (Glen Research, Sterling, Va.) was coupled as the last phosphoramidite to the 5′-end of a growing oligonucleotide chain. After cleavage from the solid support and base deprotection, the disulfide modified enzymatic nucleic acid molecule 31 (FIG. 10) was purified using ion exchange chromatography. The thiol group was unmasked by reduction with dithiothreitol (DTT) to afford 32 which was purified by gel filtration and immediately conjugated with 30. The resulting conjugate 33 was separated from the excess folate by gel filtration and then purified by RP HPLC using gradient of acetonitrile in 50 mM triethylammonium acetate (TEAA). Desalting was performed by RP HPLC. Reactions were conducted on 400 mg of disulfide modified enzymatic nucleic acid molecule 31 to afford...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
| enzymatic nucleic acid | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com