Process for the production of methanol including one or more membrane separation steps
a technology of methanol and membrane separation, which is applied in the direction of separation process, oxygen-containing compound preparation, oxygen-containing compound purification/separation, etc., can solve the problems of unnecessary costs, and achieve the effect of preventing its release to the environment and reducing the compression requirements of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Conventional Methanol Production Process (not in Accordance with the Invention)
[0084]The computer calculations in the following Examples were performed using a modeling program, ChemCad 5.6 (ChemStations, Inc., Houston, Tex.) containing code developed by assignee's engineering group for applications specific to assignee's processes.
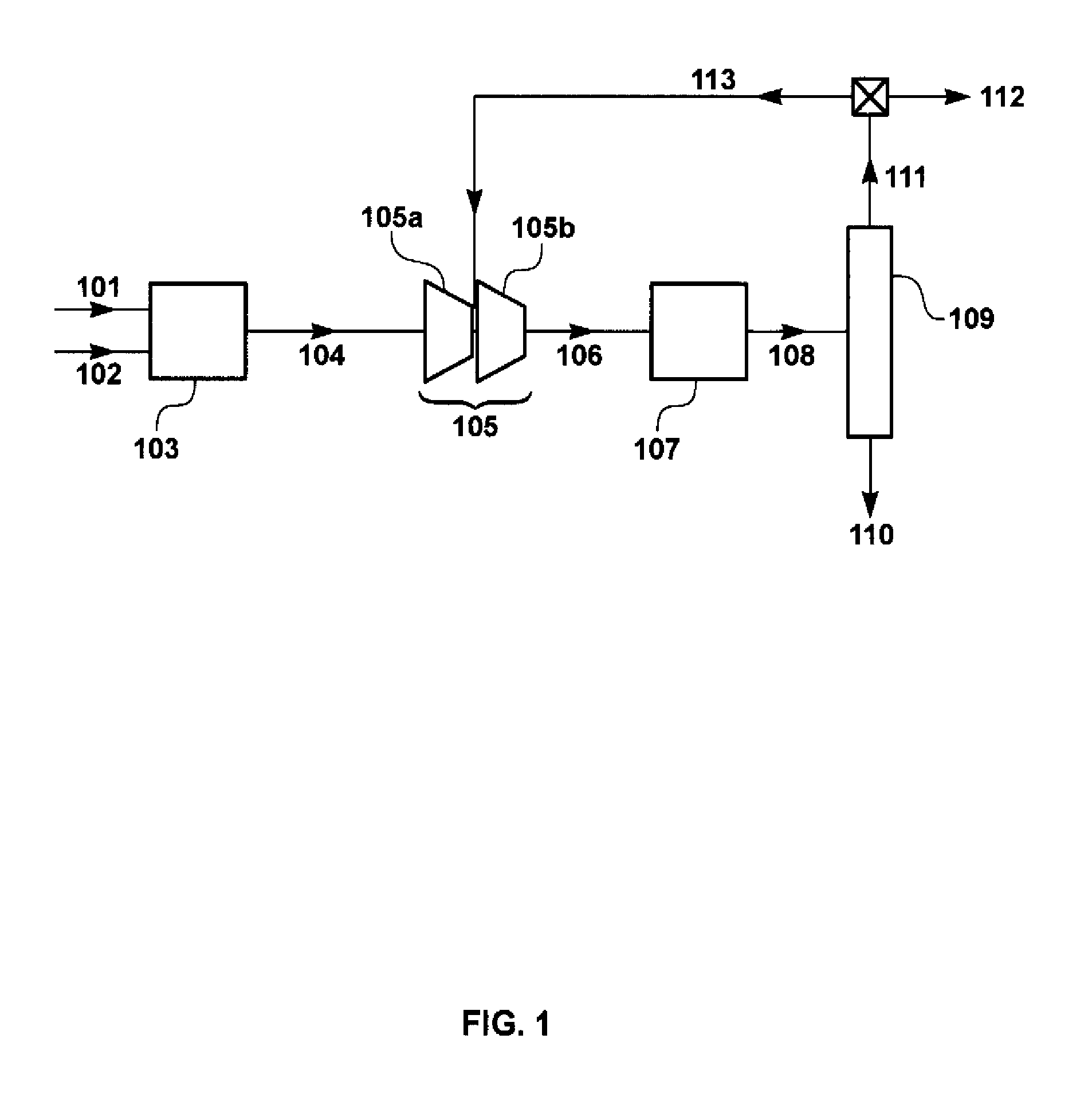
[0085]The calculation for this Example was performed using the flow scheme shown in FIG. 1 and described in the Background of the Invention, above. This flow scheme does not include a membrane separation step upstream of the methanol synthesis process (not in accordance with the invention). Syngas flow was assumed to be 106 metric tons per hour (Mt / h).
[0086]The flow rates and chemical compositions of the streams in the methanol synthesis loop were calculated. The results of this calculation are shown in Table 1.
[0087]
TABLE 1ReactorReactorOverheadRecycleSyngasFeed GasOutputCondensateStreamPurge GasGasParameter / Stream104106108110111112113Total Flow (Mt / h)10...
example 2
Methanol Production Process in Accordance with the Invention
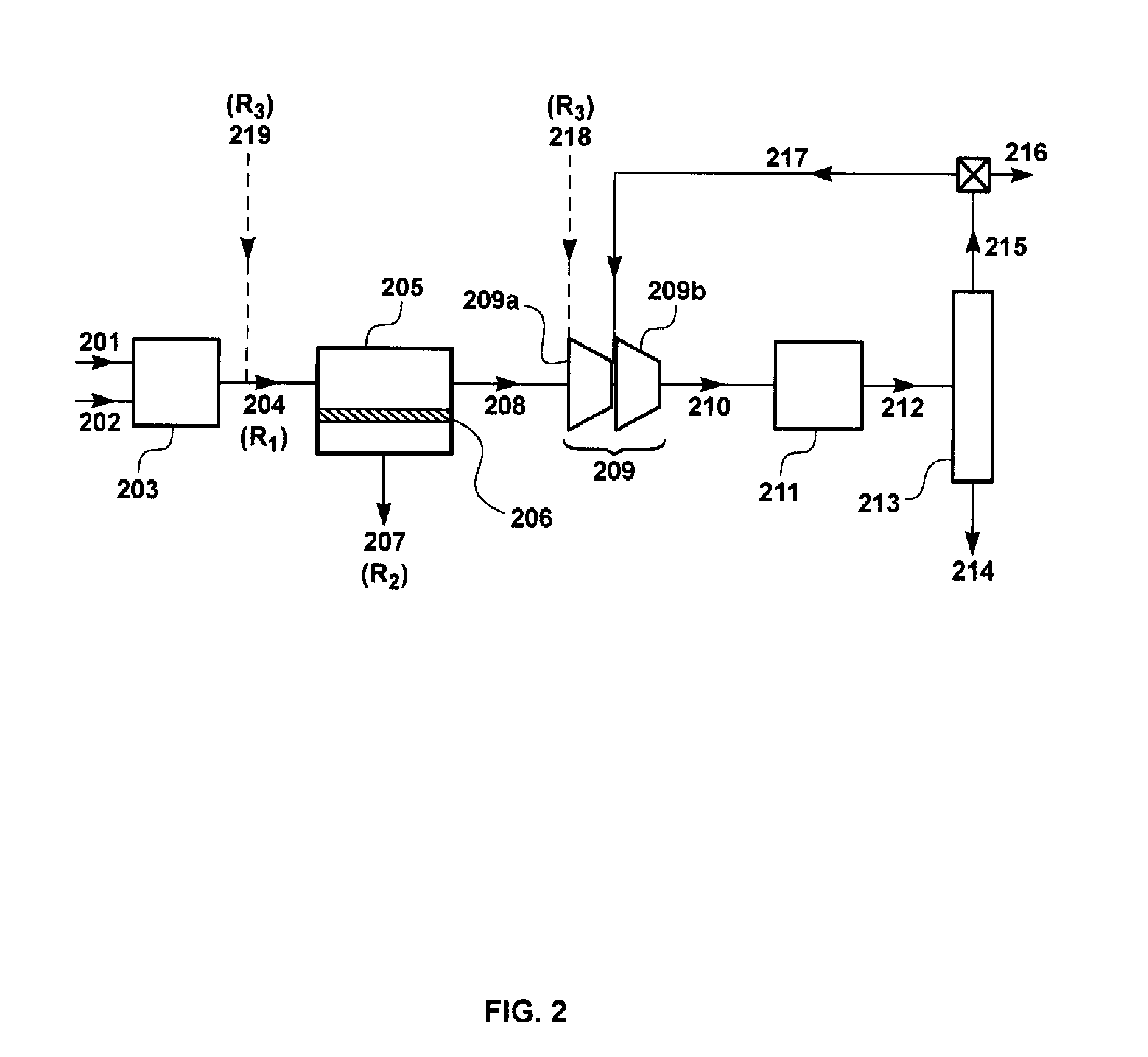
[0089]The calculation for this Example was performed using the flow scheme shown in FIG. 2 and described in the Detailed Description, above. This flow scheme includes a membrane separation step upstream of the methanol synthesis loop.
[0090]The membranes, 206, in membrane separation unit, 205, were assumed to have the properties shown in Table 2, at a membrane operating temperature within the range of about 50° C. and about 150° C.
[0091]
TABLE 2GasPermeance (gpu)*H2 / Gas Selectivity**Hydrogen300—Carbon monoxide>100Carbon dioxide2015Methane>100Nitrogen>100Water5000.6*Gas permeation unit; 1 gpu = 1 × 10−6 cm3(STP) / cm2 · s · cmHg**Estimated, not measured
[0092]As with Example 1, syngas flow for this calculation was assumed to be 106 Mt / h. The flow rates and chemical compositions of the streams in the methanol synthesis loop were calculated. The results of this calculation are shown in Table 3.
[0093]
TABLE 3ReactorMembraneTreatedFee...
example 3
Methanol Production Loss from Co-Permeation of Carbon Dioxide
[0096]Many hydrogen-permeable membranes show good selectivity for hydrogen over carbon monoxide. However, good selectivity for hydrogen over carbon dioxide is much harder to realize. Because of this, a series of calculations of the type described in FIG. 2 was performed, varying the hydrogen / carbon dioxide selectivity from 3 to 15. The results of the calculations were used to create the curves shown in FIG. 4, which is a plot 400 showing methanol production loss (due to co-permeation of carbon oxides) 401 as a function of membrane hydrogen / carbon dioxide selectivity 402. Curve 403 represents a permeate stream pressure of 4 bar (60 psia); curve 404 represents a permeate stream pressure of 2 bar (30 psia). Feed stream pressure in both cases was 240 psia.
[0097]As can be seen from the figure, at a given membrane selectivity, methanol production loss from co-permeation of carbon oxides is slightly higher at a permeate pressure ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com