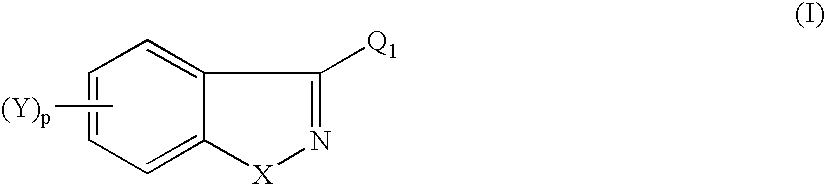
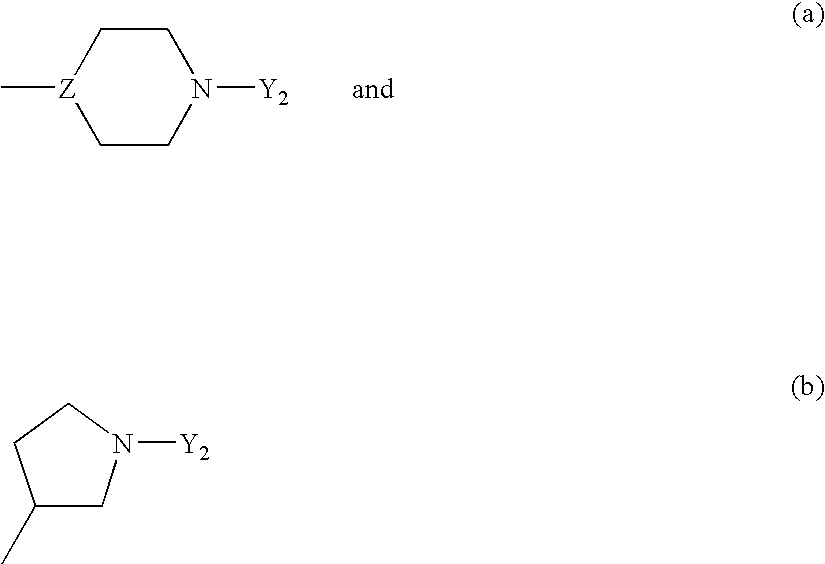
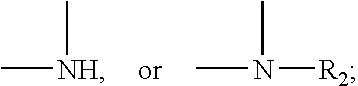Heteroarylpiperidines, pyrrolidines and piperazines and their use as antipsychotics and analgesics
a technology of pyrrolidine and piperazine, which is applied in the direction of ketone active ingredients, group 4/14 element organic compounds, drug compositions, etc., can solve the problems of known neuroleptics producing unwanted side effects, not treating psychotic patients, and almost certainly relaps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 1-[4-[3-[4-(1H-Indazol-3-yl)-1-piperazinyl]propoxy]-3-methoxyphenyl]ethanone
(A) Synthesis of 3-bromobenzoic acid 2-phenylsulfonylhydrazide
[0424]To a solution of 2-bromobenzoic acid, hydrazide (132 g) in pyridine (1.2 l) cooled to about 10° with an ice bath, was added benzenesulfonyl chloride (7.3 ml). After complete addition, the reaction was stirred at ambient temperature for four hours, and then poured into ice-hydrochloric acid to precipitate a yellow solid, 135 g. The material was recrystallized from isopropanol to yield 125 g of 2-bromobenzoic acid 2-phenylsulfonylhydrazide, m.p.=154°-156° C.
(B) Synthesis of α-chloro-2-bromobenzaldehyde phenylsulfonylhydrazone
[0425]A mixture of 2-bromobenzoic acid phenylsulfonylhydrazide (125 g, (1.35 mol) and thionyl chloride (265 ml) was stirred and refluxed for 2hours. After about 15 minutes of reflux, the solid went into solution. The reaction was permitted to cool, and then it was poured into hexane. The resultant white soli...
example 2
1-[4-[3-[4-(1,2-Benzisoxazol-3-yl)-1-piperadinyl]propoxyl]-3-methoxyphenyl]ethanone
[0445]A mixture of 3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride (4.8 g, 0.02 mol), K2CO3 (5.2 g, 0.04 mol), 1-[4-(3-chloropropoxy)-3-methoxyphenyl]ethanone (5.3 g, 0.022 mol), a few crystals of KI and dimethylformamide (60 ml) was stirred at 90° C. for 16 hours. The reaction was poured into water and the aqueous mixture was extracted with ethyl acetate. The extract was washed (water), dried (MgSO4) and concentrated to afford a brown oil. The oil was chromatographed on a Waters Prep 500 utilizing silica gel columns and ethyl acetatediethylamine (2%), eluent. Concentration of the appropriate fractions afforded 3.9 g of product as an off-white solid. Recrystallization from absolute ethyl alcohol afforded 26 g (33%) of 1-[4-[3-[4-(1,2-benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3-methoxy-phenyl]ethanone, m.p.=102°101° C., as colorless needles.
ANALYSIS
[0446]Calculated for C24H28N2O4: 70.56% C 6.91% H 6....
example 3
1-[4-[3-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)-1-piperadinyl]propoxy]-3-methoxyphenyl]ethanone
[0448]A stirred mixture of 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride (5.1 g, 0.02 mol), K2CO3 (5.2 g. 0.04 g, mol), 1-(4-(3-chloropropoxy-3-methoxyphenyl]ethanone (5.3 g, 0.022 mol), and dimethylformamide (60 ml) was heated at 90° C. for 16 hour. The reaction was poured into water, and the aqueous mixture was extracted with ethyl acetate. The ethyl acetate was washed (water), dried (MgSO4) and concentrated to afford a moist solid. Recrystallization (twice) from ethyl alcohol afforded 5.0 (58%) of 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3methoxyphenyl]ethanone as a beige solid, m.p.=118°-120° C.
ANALYSIS
[0449]Calculated for C24H27FN2O4: 67.60% C 6.38% H 6.57% N.
[0450]Found: 67.47% C 6.40% H 6.53% N.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com