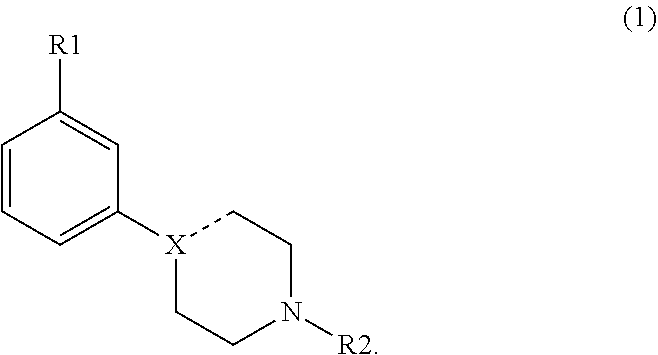
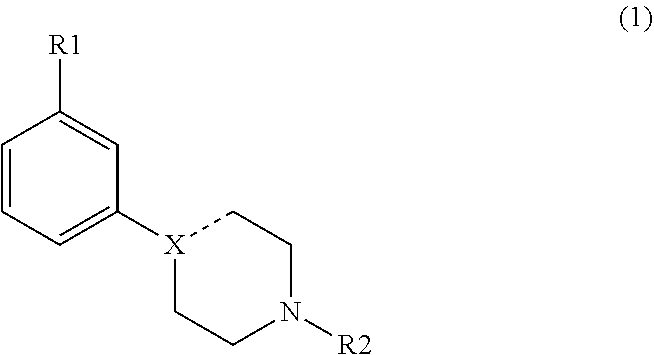
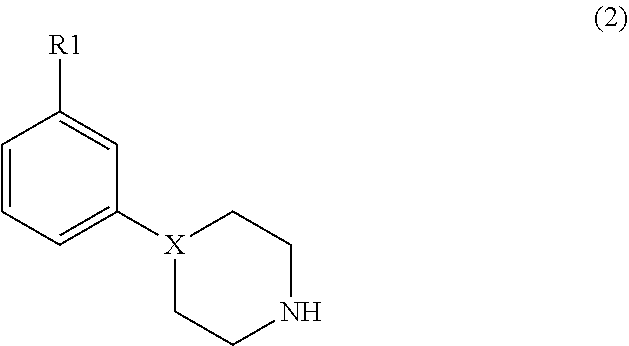
Modulators of dopamine neurotransmission
a technology of dopamine neurotransmission and modulators, applied in the field of new modulators of dopamine neurotransmission, can solve problems such as inability to obtain, and achieve the effects of high oral bioavailability and good pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1-(3-Methanesulfonyl-phenyl)-4-propyl-pierazine
[0123]A suspension of 1-(3-methanesulfonyl-phenyl)-piperazine (350 mg) and ground K2CO3 (403 mg) was stirred in CH3CN (25 mL) at room temperature. 1-Iodopropane (712 μL) was added. The mixture was refluxed overnight. The reaction mixture was filtered and the volatiles were evaporated in vacuum. The oily residue was chromatographed on a silica column with MeOH:CH2Cl2 (1:30 (v / v)) as eluent. Collection of the fractions containing pure product and evaporation of the solvent afforded pure 1-(3-methanesulfonyl-phenyl)-4-propyl-piperazine (220 mg). The amine was converted into the HCl salt and recrystallized from ethanol / diethylether: m.p. 233° C. MS m / z (relative intensity, 70 eV) 282 (M+, 30), 254 (15), 253 (bp), 210 (17), 70 (21).
[0124]The following compounds according to Examples 2-11 were prepared in a manner similar to the one described in Example 1.
example 2
1-Propyl-4-(3-Trifluoromethanesulfonyl-phenyl)-piperazine
[0125]MS m / z (relative intensity, 70 eV) 336 (M+, 16), 307 (bp), 77 (18), 70 (38), 56 (23).
example 3
1-[3-(4-Propyl-piperazin-1-yl)-phenyl]-ethanone
[0126]Beginning with 1-(3-Piperazin-1-yl-phenyl)-ethanone and n-Pr-I: m.p. 119° C. (oxalate), MS m / z (rel. intensity, 70 eV) 246 (M+, 10), 217 (33), 132 (18), 70 (bp), 56 (41); Rf 0.23 (EtOAc).
PUM
| Property | Measurement | Unit |
|---|---|---|
| DA autoreceptor agonist properties | aaaaa | aaaaa |
| DA autoreceptor agonist properties | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com