Tissue specific expression of retinoblastoma protein
A technology for expressing vectors and fusion polypeptides, applied in the direction of retinoblastoma protein, mammalian protein, peptide/protein components, etc., which can solve the problems of cell growth stagnation and unclear exact pathway of arrest
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
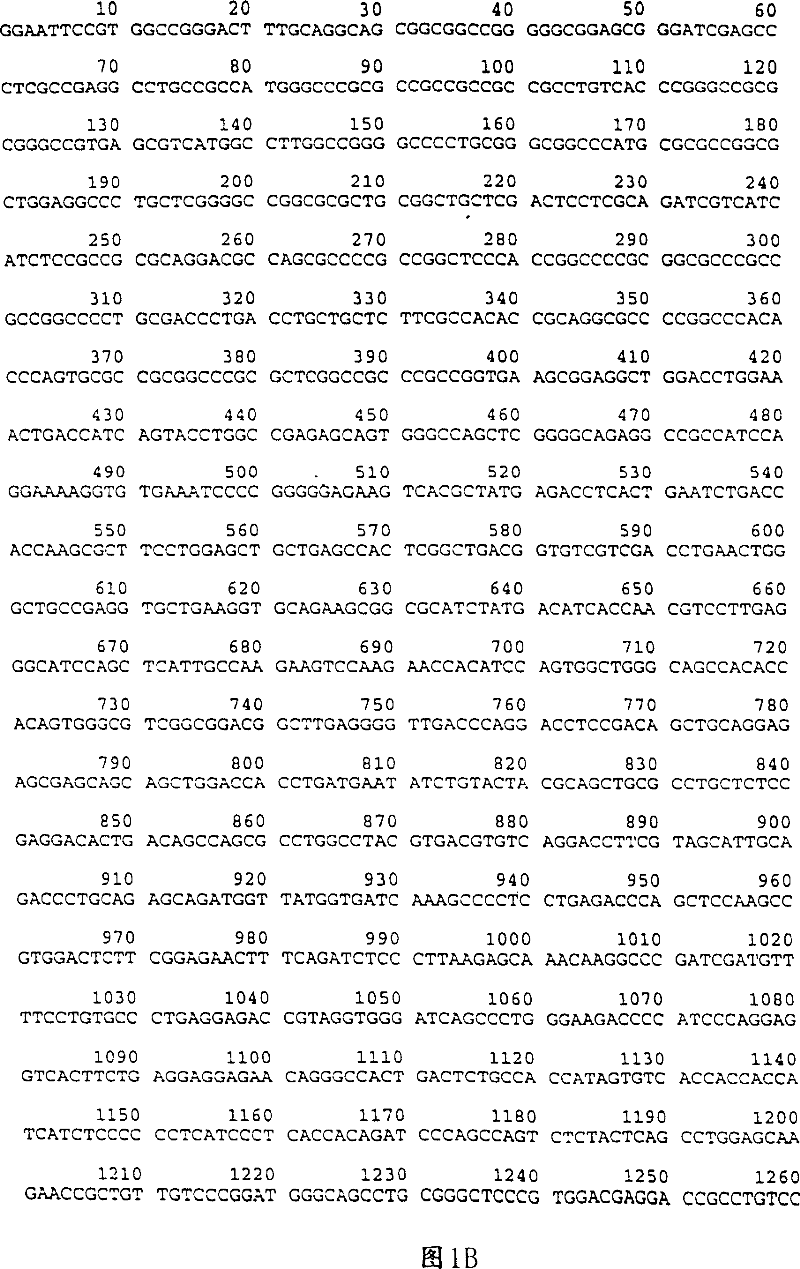
[0081] E2F-RB fusion
[0082] A. Introduction
[0083] In this example, expression plasmids encoding different segments of E2F fused to RB56 polypeptides were constructed. RB56 is a subfragment of full-length RB that contains the "pocket" domain necessary for growth inhibition (Hiebert et al. MCB 13:3384-3391 (1993); Qin et al. Genes and Rev. .6: 953-964 (1992)). E2F194 comprises E2F amino acids 95-194. This fragment contains only the DNA-binding domain of E2F. E2F286 contains a DNA binding domain and a DP-1 heterodimerization domain. Both E2F fragments lack the N-terminal cyclin A-kinase-binding domain, which appears to downregulate the DNA-binding activity of E2F (Krek et al. Cell 83:1149-1158 (1995); Krek et al., Cell 78:161-172 (1994)).
[0084] B. Construction of the vector
[0085] Plasmid pCTM contains a CMV promoter, a triple adenoviral leader flanked by T7 and SP6 promoters, a multiple cloning site, and downstream of the multipl...
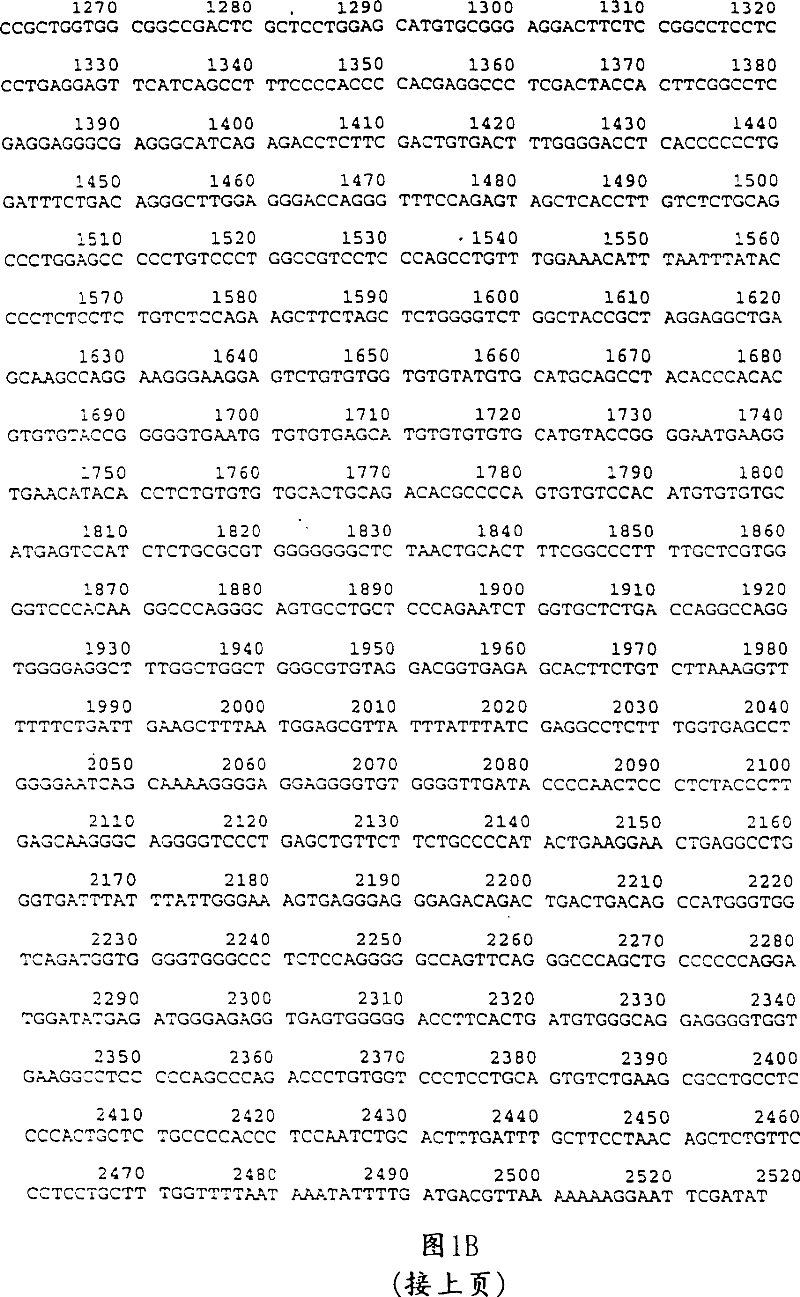
Embodiment II
[0108] Tissue-specific expression of E2F-RB fusions
[0109] A. Construction of recombinant adenovirus
[0110] In this experiment, recombinant adenoviruses containing the RB polypeptide under the control of a CMV promoter or the smooth muscle alpha-actin promoter were generated.
[0111]Isolation of the smooth muscle α-actin promoter (bases -670 to +5) by PCR from a genomic library, Reddy et al., "Construction of the human smooth muscle α-actin gene" J. Biol. Chems .265: 1683-1687 (1990), Nakano et al., "Transcriptional regulatory elements and the first intron 5' upstream of the human smooth muscle (aortic type) α-actin-encoding gene" Gene 99:285-289 (1991)), and added 5' Xho I and Avr II and 3' Xha I, Cla I and Hind III restriction enzyme sites for cloning purposes. The fragment was subcloned into a plasmid as an Xho I, Hind III fragment and sequenced to confirm its base composition. A fusion construct 286-56 was subcloned as an Xha I, Cla I fragment di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com