Medical coating system having double-term and/or multi-term release speed rate
A technology of coating system and release rate, applied in coatings, medical science, catheters, etc., can solve the problems of inability to prevent infection, and achieve good anti-infection, strong hydrophilicity, and slow release rate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
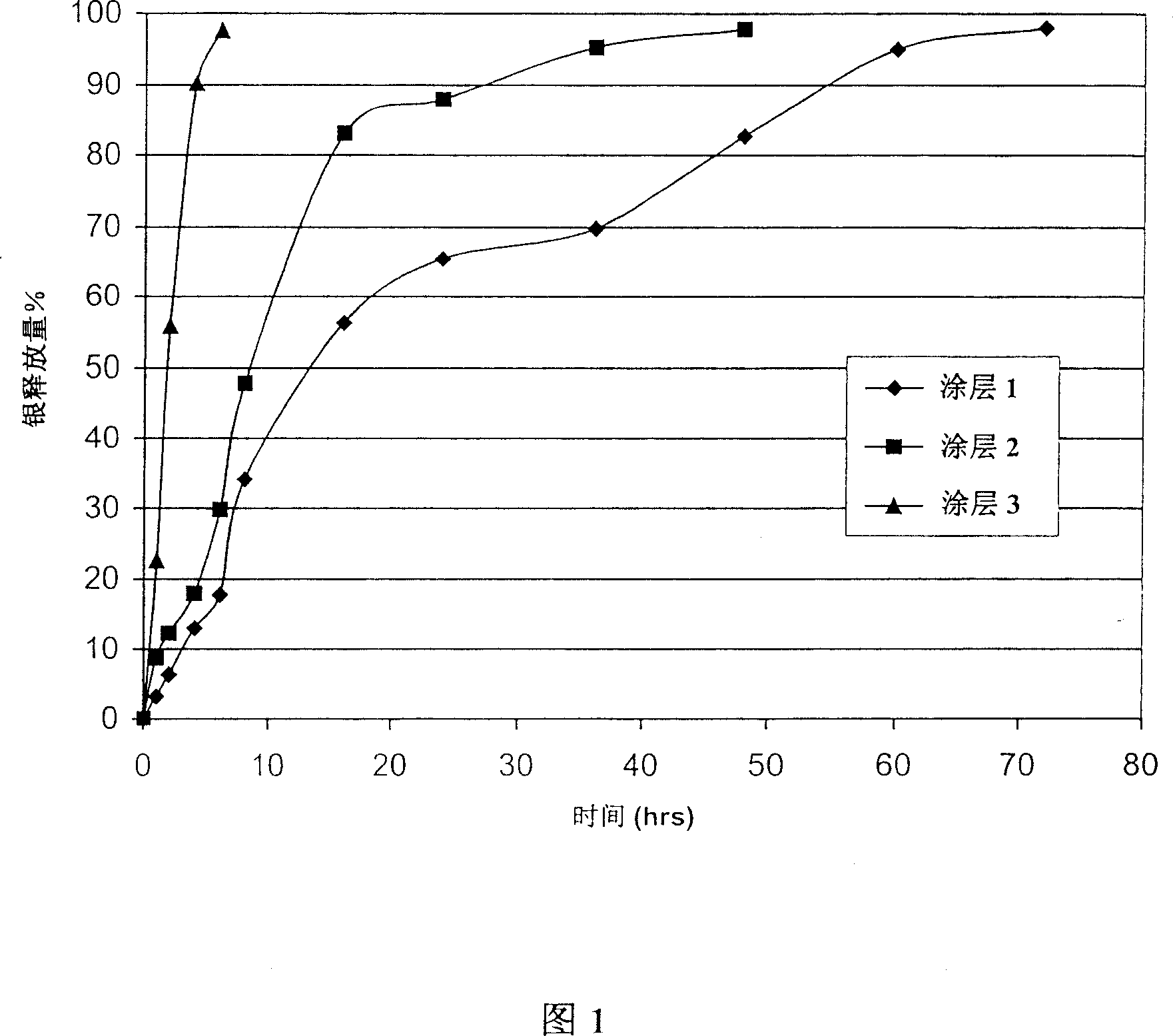
[0047] The preparation of three different molecular weight polyethylene oxide coating liquids.
[0048] Coating liquid 1: PEO (molecular weight 4×10 6 , Union Carbide Corp.) is 2%, silver oxide (sold by Sigma) is 0.2%. PEO and silver oxide are uniformly mixed in isopropanol / water (80:20) solution to prepare coating liquid 1.
[0049] Coating liquid 2: PEO (molecular weight 3×10 5 , Union Carbide Corp.) is 3%, silver oxide (sold by Sigma) is 0.2%. PEO and silver oxide are uniformly mixed in isopropanol / water (80:20) solution to prepare coating liquid 2.
[0050] Coating liquid 3: PEO (molecular weight 2×10 4 , Union Carbide Corp.) is 8%, silver oxide (sold by Sigma) is 0.2%, PEO and silver oxide are uniformly mixed in isopropanol / water (80:20) solution to prepare coating liquid 3.
[0051] The three latex catheters were respectively immersed in the above three coating solutions, and then dried in an oven at 80° C., and the above steps were repeated once to form three polyethylene o...
Embodiment 2
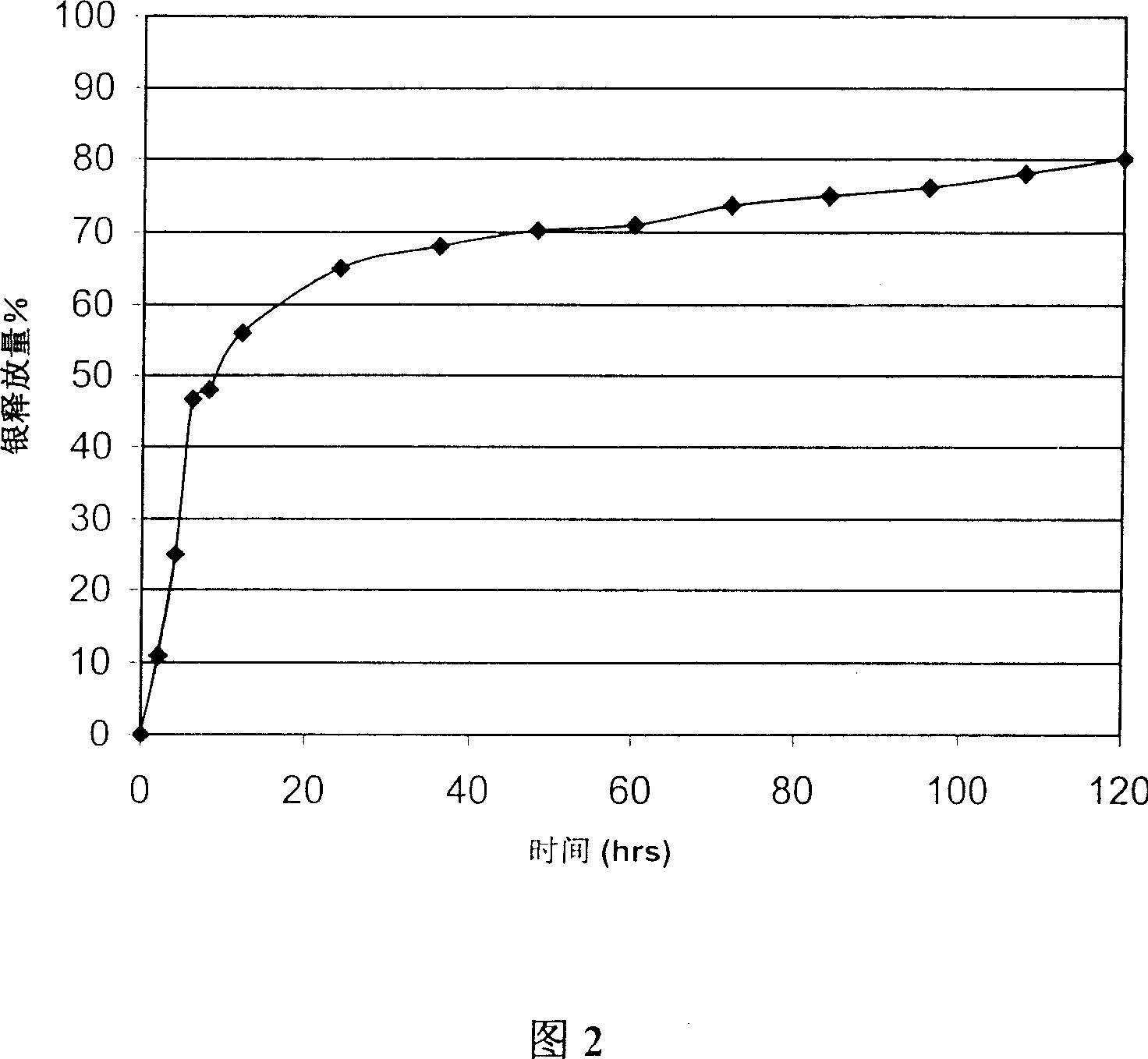
[0055] A two-layer coating system with a base layer and an outer layer is formed on the latex catheter.
[0056] First, the coating solution for the base layer and outer layer of the catheter is prepared as follows.
[0057] Base layer: PEO (molecular weight 4×10 6 , Union Carbide Corp.) is 2%, and silver oxide is 0.2% (sold by Sigma). PEO and silver oxide are uniformly mixed in a solution of isopropanol / water (80:20) and added to the solution In the stoichiometric ratio of ethyleneimine to PEO of 1:1, ethyleneimine is added to form a base layer coating liquid.
[0058] Outer layer: PEO (molecular weight 2×10 4 , Union Carbide Corp.) is 8%, and silver oxide (sold by Sigma) is 0.2%. PEO and silver oxide are uniformly mixed in an isopropanol / water (80:20) solution.
[0059] Then, the latex catheter is first immersed in the base layer coating solution, dried at 80°C, then dipped in the outer layer coating solution, and dried at 80°C to form a base layer and outer layer double-layer c...
Embodiment 3
[0063] The anti-infective effect test of the catheter with the coating system of the present invention.
[0064] For the catheter section of the double-layer coating system with a base layer and an outer layer formed in Example 2, a bacteriostatic zone determination test (without a silver release test) was performed to test its anti-infection effect.
[0065] In the inhibition zone test, the test medium is Muller=Hinton agar, and the test bacteria is E. coli. The zone of inhibition after 24 hours was 8.5 mm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com