Compound 2-methylol-3-substituted phenyl propionic acid with optical activity and its resolving process
A technology of phenylpropionic acid and hydroxymethyl, which is applied in the chemical field, can solve the problems of cumbersome steps, high cost, and unsuitability for industrial production, and achieve the effects of easy recycling, low price, and good separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
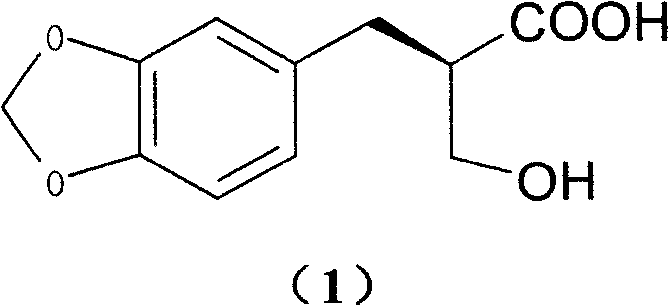
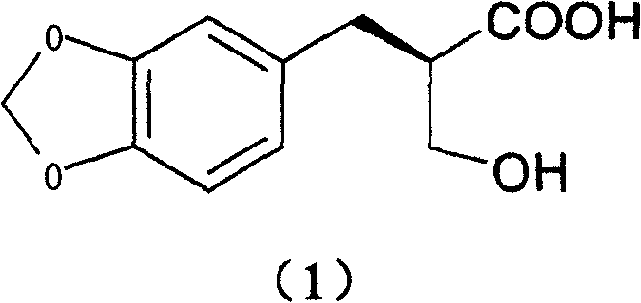
[0032] Add 4.0g (17.9mmol) of 2-hydroxymethyl-3-(3,4-methylenedioxy)phenylpropionic acid and 3.5g (17.9mmol) of meglumine into a 250ml single-necked bottle, add 60ml of ethanol, After reflux for 10 minutes, it was naturally cooled to room temperature, allowed to stand for 2 days, and 3.6 g of solid was obtained by filtration. Then recrystallized once with 100ml of ethanol to obtain 3.3g of solid.
[0033] Add 50ml of water to the solid, adjust the pH to 9 with 10% NaOH aqueous solution while stirring, filter, adjust the pH to 2 with 10% dilute hydrochloric acid in the filtrate, extract with 80ml ethyl acetate, dry over anhydrous sodium sulfate, and concentrate. Stir and crystallize with 100ml of petroleum ether to obtain 1.6g of white solid (40.0% yield), mp90°C. [α] D 20 =+29.4 (c=1, methanol)
Embodiment 2
[0035] Add 4.0g (17.9mmol) of 2-hydroxymethyl-3-(3,4-methylenedioxy)phenylpropionic acid and 5.2g (17.9mmol) of octylglucamine into a 250ml single-necked bottle, add 120ml of ethanol, After reflux for 10 minutes, it was naturally cooled to room temperature, allowed to stand for 2 days, and 3.6 g of solid was obtained by filtration. Then 100ml of ethanol was used to recrystallize 4 times to obtain 3.3g of solid.
[0036] Add 50ml of water to the solid, adjust the pH to 9 with 10% NaOH aqueous solution while stirring, filter, adjust the pH to 2 with 10% dilute hydrochloric acid in the filtrate, extract with 80ml ethyl acetate, dry over anhydrous sodium sulfate, and concentrate. Stir and crystallize with 100ml of petroleum ether to obtain 1.4g of white solid (yield 35.2%)
Embodiment 3
[0038] Add 4.0g (17.9mmol) of 2-hydroxymethyl-3-(3,4-methylenedioxy)phenylpropionic acid and 2.16g (17.9mmol) of (+)-α-phenylethylamine into a 100ml single-necked bottle 50ml of ethanol was added, refluxed for 10 minutes, cooled to room temperature naturally, left standing for 2d, and filtered to obtain 3.67g of solid. Then recrystallized twice with 30ml of ethanol to obtain 3.2g of solid.
[0039] Add 50ml of water to the solid, adjust the pH to 2 with 10% dilute hydrochloric acid under stirring, filter, and dry in vacuo to obtain 1.35g of white solid (yield 33.8%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com