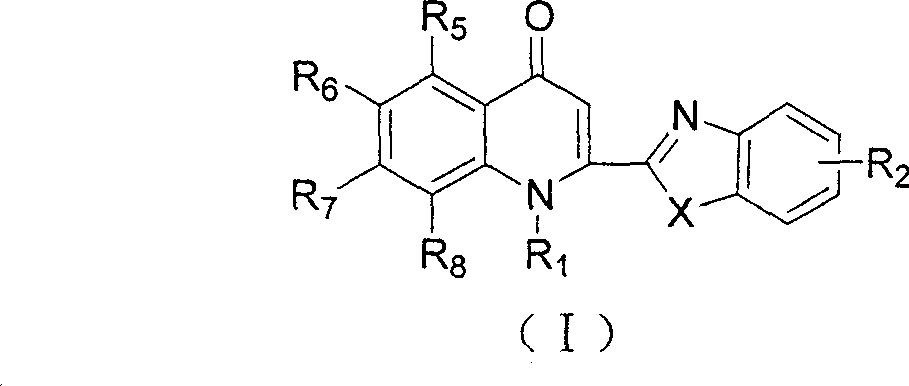
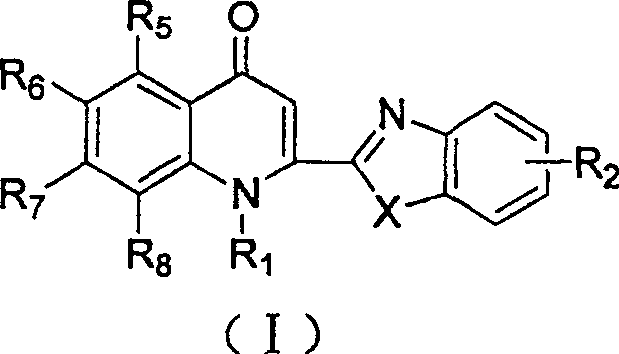
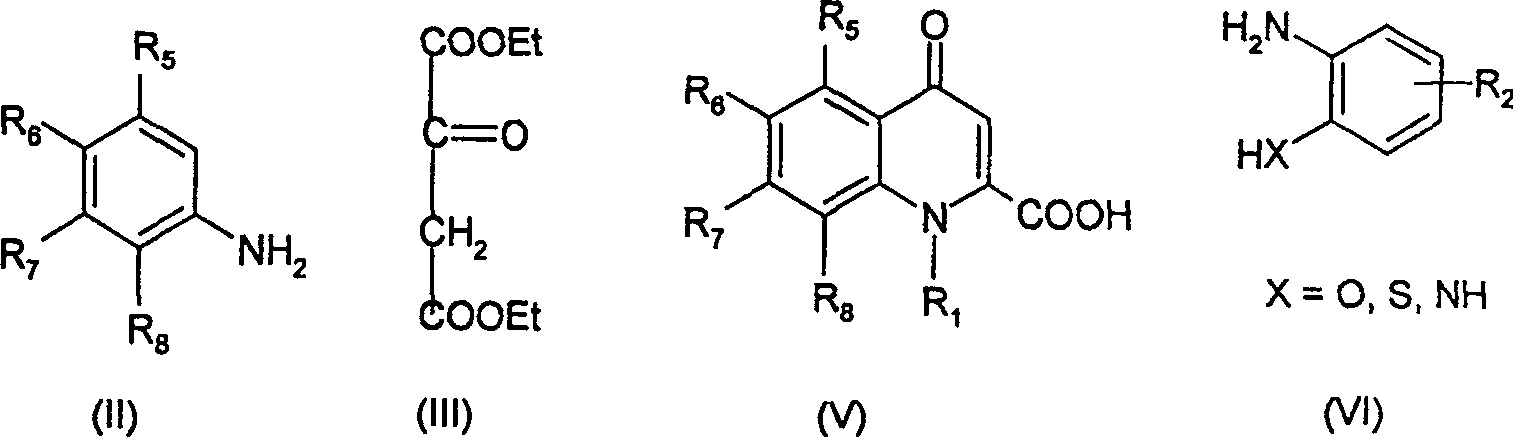
2-substituted quinolone compound and use in pharmacy
A technology of compounds and general formulas, applied in the field of pharmaceuticals, can solve problems such as undisclosed quinolones
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of Diethyl Butanonedioate
[0052] Add absolute ethanol (50ml) and sodium (10g, 0.435mol) to a 500ml three-necked flask equipped with a reflux condenser and a drying tube, and heat to reflux until the sodium disappears after the reaction is slightly slow. Change the reflux device to a distillation device, evaporate the ethanol to dryness under reduced pressure, and release the vacuum after cooling to room temperature. Add anhydrous ether (80ml) and stir to form a paste, cool to below -5°C, slowly add diethyl oxalate (58.23ml, 0.427mol) and ethyl acetate (39.3ml, 0.401mol) dropwise below -5°C Mixture. Stirring was continued for 1 hour, then the temperature was raised to 38-40° C., refluxed for 1 hour, and left overnight. Neutralize to pH 1-2 with 10% sulfuric acid at low temperature, separate the ether layer, wash with water several times, wash with saturated sodium bicarbonate solution three times, then wash with saturated brine, anhydrous Na 2 SO 4 dry....
Embodiment 2
[0054] 1,4H-quinolin-4-one-2-carboxylic acid ethyl ester
[0055] Aniline (14g, 0.15mol), diethyl butanonedioate (29g, 0.154mol), and benzene (20ml) were added to the reaction flask, and refluxed for 3 hours under nitrogen protection. Benzene was recovered, and silica gel column chromatography was carried out using a mixed solvent of ethyl acetate and petroleum ether as a developing solvent to obtain a yellow liquid. The resulting yellow liquid was mixed with diphenyl ether (60ml), heated to reflux and kept at that temperature for 1 hour. After cooling, a pale yellow solid was precipitated, filtered, washed with petroleum ether, and dried to obtain 11.7 g of the product, yield 35.9%, mp 209-211°C.
Embodiment 3
[0057] 1,4H-quinolin-4-one-2-carboxylic acid
[0058] A mixture of ethyl 1,4H-quinolin-4-one-2-carboxylate (11.5 g, 0.053 mol), sodium hydroxide (4.24 g, 0.016 mol), and water (57 ml) obtained in Example 2 was refluxed for 2 hours. Cool, filter, wash with water, and dry to obtain 9.1 g of off-white powder, yield 90.8%, mp 279°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com