Preparing method for controlled released type tablet tamsulosin hcl and the tablet thereof
A technology for sustained-release tablets and hydrochloric acid, which is applied in the field of preparing tamsulosin hydrochloride sustained-release tablets and tamsulosin hydrochloride sustained-release tablets, and achieves the effects of high yield, high economic factor and uniform distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
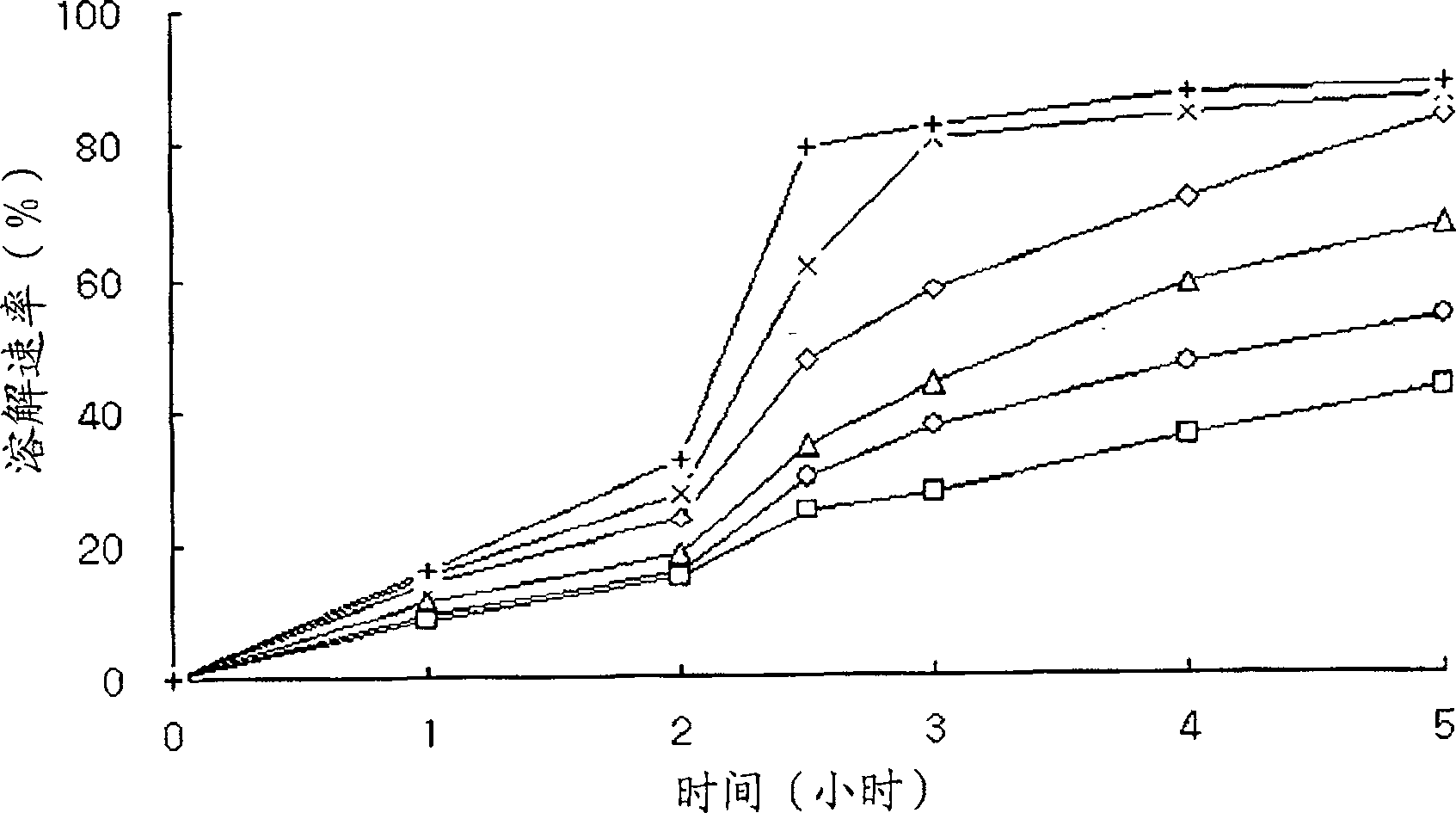
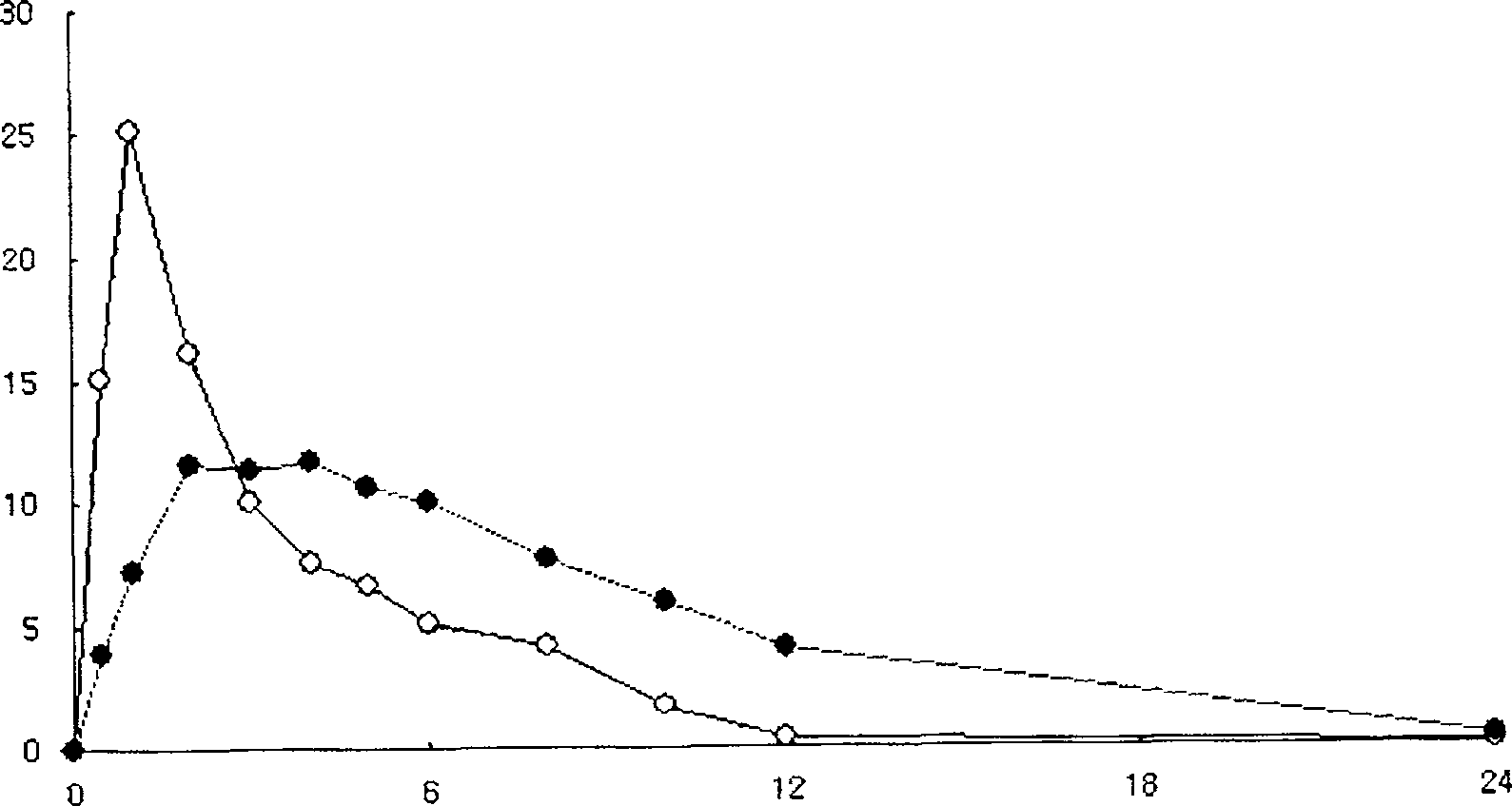
Embodiment 1
[0052] Sustained-release tablets containing the following ingredients were prepared as follows:
[0053] Tamsulosin Hydrochloride 0.2g
[0054] Hydroxypropylmethylcellulose phthalate (A) 10g
[0055] Hydroxypropylmethylcellulose phthalate (B) 65g
[0056] Lactose 99.8g
[0057] Glyceryl Dielmate 15g
[0058] Metolose 60SH-4000(A)15g
[0059] Metolose 60SH-4000(B)20g
[0061] A binder solution was prepared by completely dissolving tamsulosin hydrochloride in 60 ml of a mixed organic solvent (ethanol:water=8:2), followed by dissolving hydroxypropylmethylcellulose phthalate (A) therein. Meanwhile, lactose, hydroxypropylmethylcellulose phthalate (B), Metolose 60SH-4000 (A) and glyceryl dielmate were mixed with each other, and the resulting binder solution was added thereto and kneaded. The kneaded material is granulated and dried. The dried granules were sieved and mixed with Metolose 60SH-4000(B) as an excipient and magnesium stearate as a l...
Embodiment 2
[0063] Sustained-release tablets containing the following ingredients were prepared as follows:
[0064] Tamsulosin Hydrochloride 0.2g
[0065] Hydroxypropylmethylcellulose phthalate (A) 10g
[0066] Hydroxypropylmethylcellulose phthalate (B) 60g
[0067] Lactose 94.8g
[0068] Glyceryl Dielmate 15g
[0069] Cereal starch 20g
[0071]A binder solution was prepared by completely dissolving tamsulosin hydrochloride in 50 ml of a mixed organic solvent (ethanol:water=8:2), and then dissolving hydroxypropylmethylcellulose phthalate (A) therein. At the same time, lactose, hydroxypropylmethylcellulose phthalate (B) and glyceryl diglycerin were mixed with each other, and the resulting binder solution was added thereto and kneaded. The kneaded material is granulated and dried. The dried granules are sieved and mixed with cereal starch as excipient and magnesium stearate as lubricant, followed by compression into tablets with a tablet machine. Each...
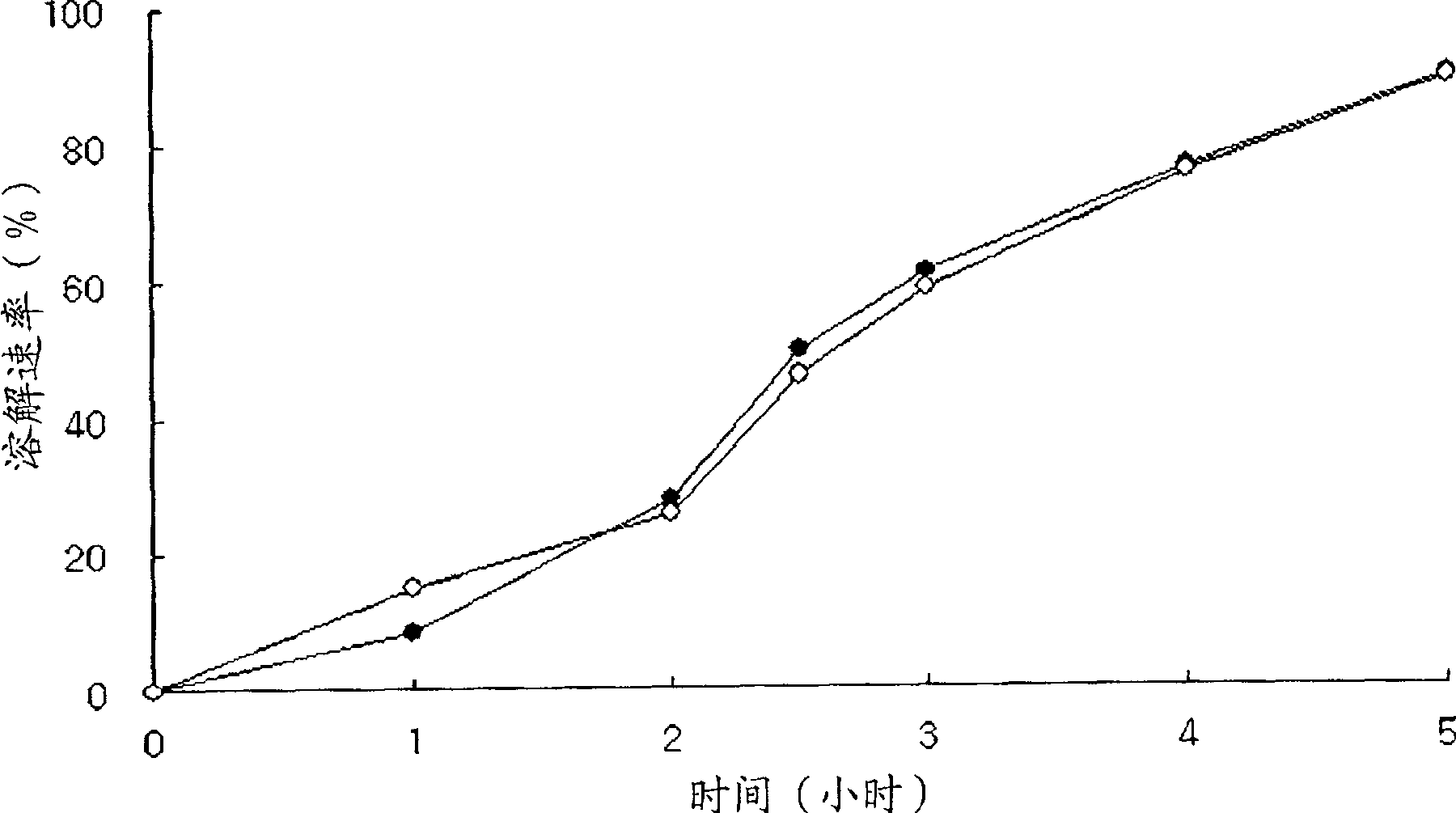
Embodiment 3
[0073] Except adding the low-substituted hydroxypropyl cellulose 11, the sustained-release tablet containing the following components was prepared in the same manner as in Example 1:
[0074] Tamsulosin Hydrochloride 0.2g
[0075] Hydroxypropylmethylcellulose phthalate (A) 20g
[0076] Hydroxypropylmethylcellulose phthalate (B) 70g
[0077] Lactose 104.8g
[0078] Glyceryl Dielmate 15g
[0079] Low-Substituted Hydroxypropyl Cellulose 1115g
[0080] Metolose 60SH-4000(B)30g
[0081] Magnesium stearate 2g
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com