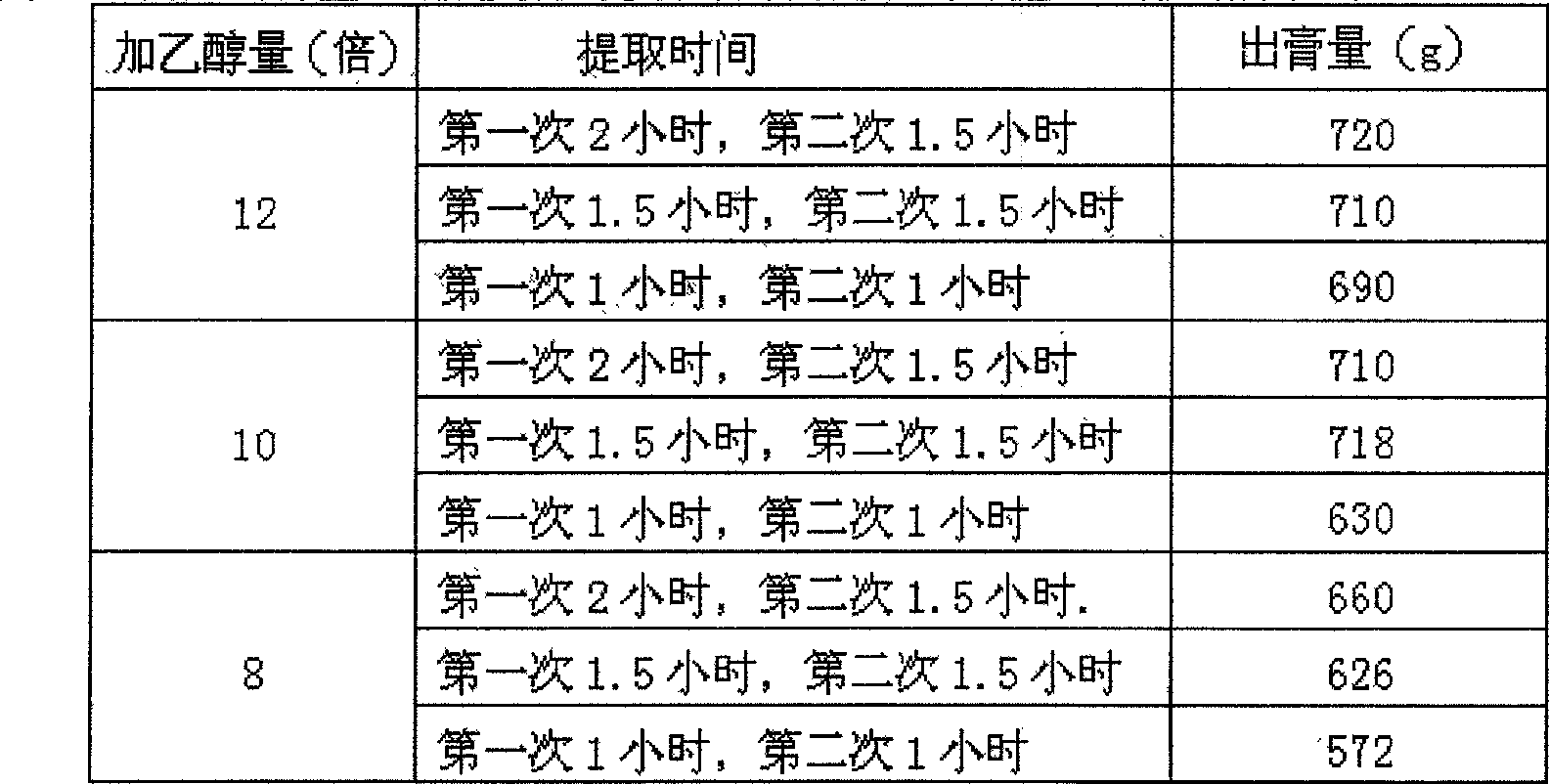
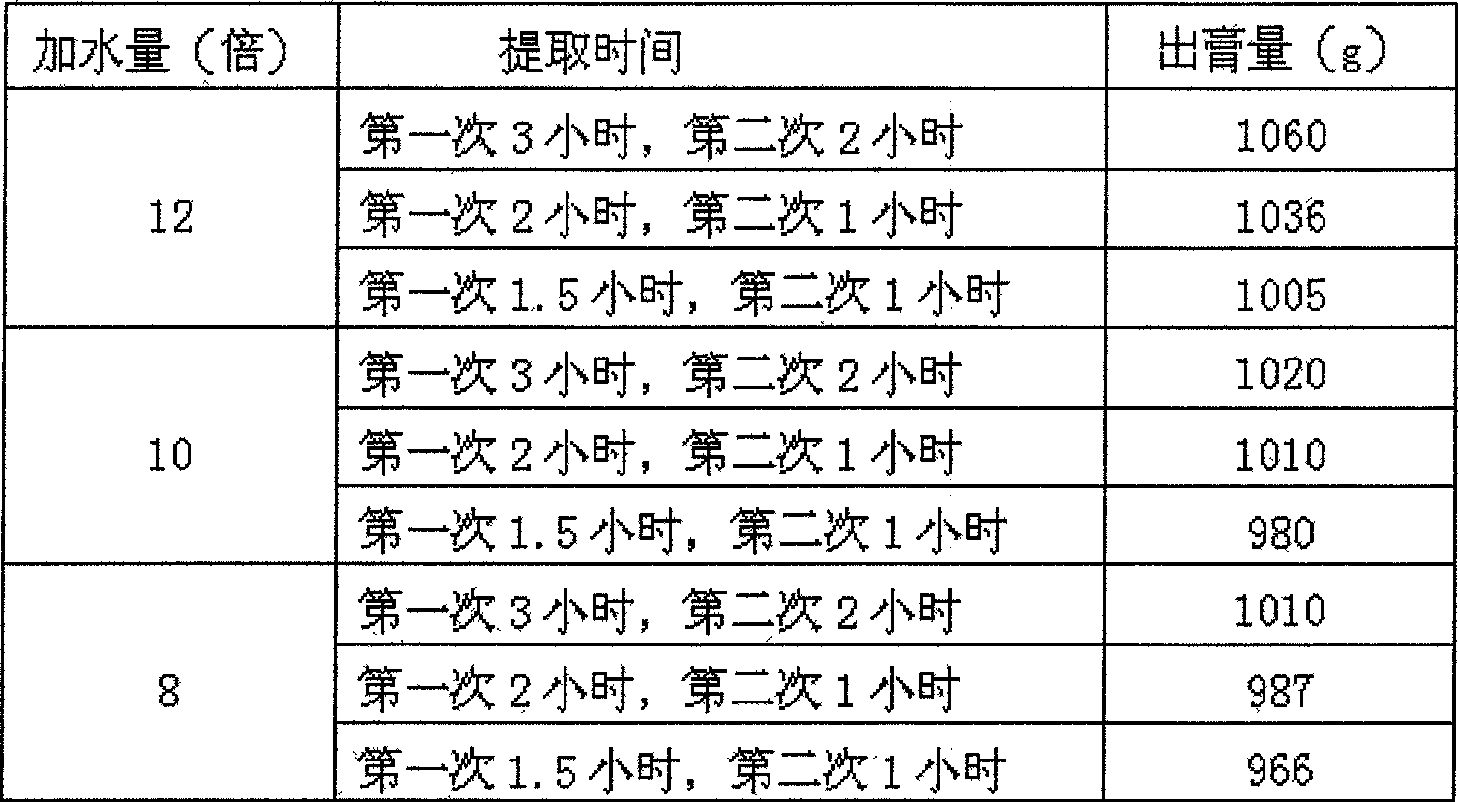
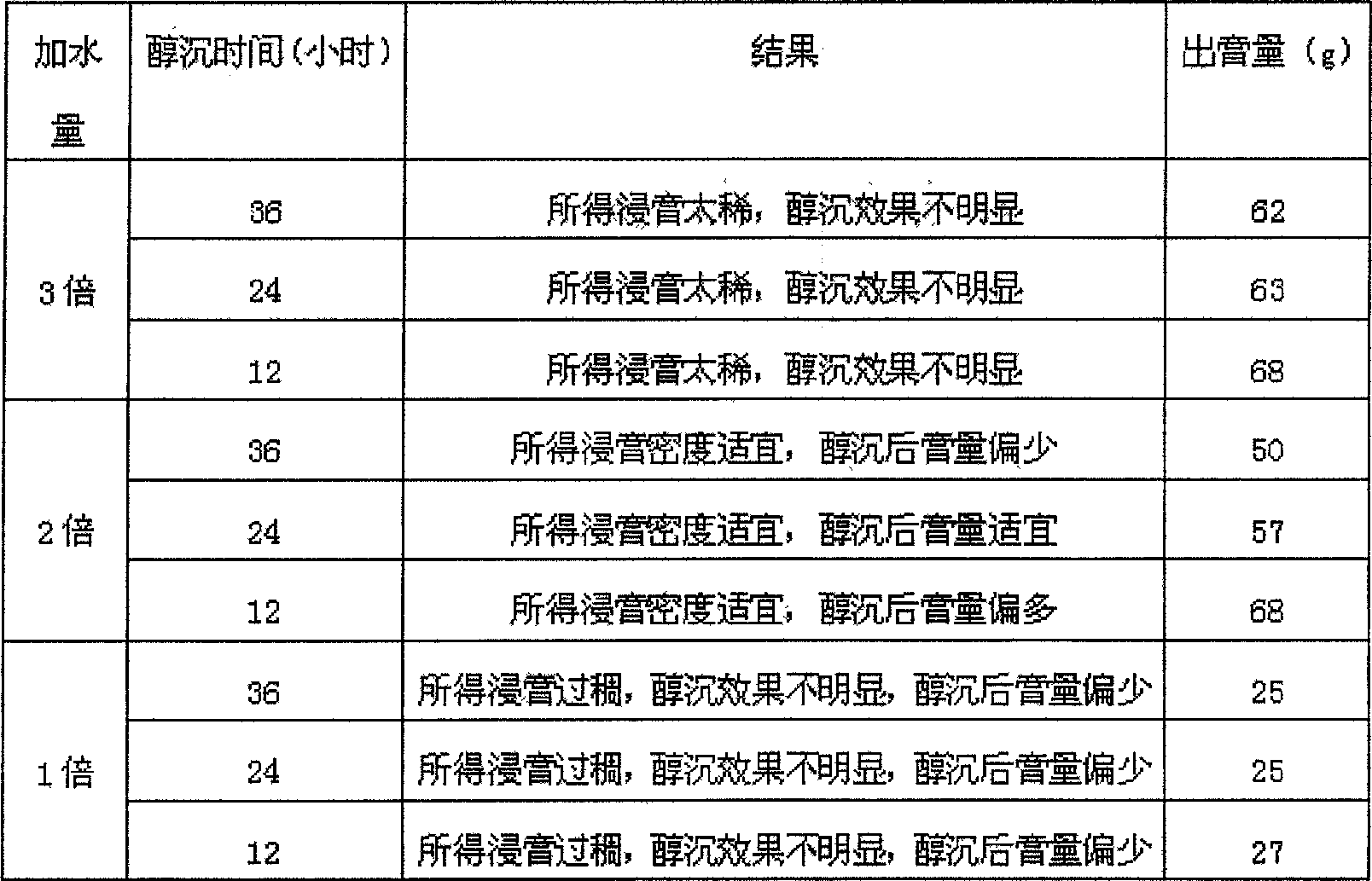
Method of testing oral disintegration tablets of 'Huo Xiang Zheng Qi'
The technology of oral disintegrating tablet and detection method is applied in the detection field of Huoxiang Zhengqi oral disintegrating tablet, and can solve the problems of slow effect, high irritation, poor taste and taste, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0205] Embodiment 2 of the present invention: described quality control method comprises the following contents:
[0206] Properties: The drug is brown to tan tablets; fragrant, pungent, slightly sweet;
[0207] Identification: (1) Take 4 tablets of this preparation, grind finely, add 20ml of n-hexane, sonicate for 15 minutes, filter, evaporate the filtrate to dryness at low temperature, add 2ml of n-hexane to the residue to dissolve, and use it as the test solution; Medicinal material 0.5g, add n-hexane 2ml, sonicate for 15 minutes, filter, and the filtrate is used as the reference medicinal material solution; according to the "Chinese Pharmacopoeia" 2005 edition, an appendix VIB thin-layer chromatography test, absorb 5 μl of each of the above two solutions, and point respectively On the same silica gel G thin-layer plate, develop with 60-90°C petroleum ether: ethyl acetate = 20:1 as the developer, take it out, dry it in the air, and spray with 5% p-dimethylaminobenzaldehyde ...
Embodiment 3
[0219] Embodiment 3 of the present invention: quality control method may comprise the following content:
[0220] Properties: The drug is brown to tan tablets; fragrant, pungent, slightly sweet;
[0221] Identification: (1) Take 4 tablets of this preparation, grind finely, add 20ml of n-hexane, sonicate for 15 minutes, filter, evaporate the filtrate to dryness at low temperature, add 2ml of n-hexane to the residue to dissolve, and use it as the test solution; Medicinal material 0.5g, add n-hexane 2ml, sonicate for 15 minutes, filter, and the filtrate is used as the reference medicinal material solution; according to the "Chinese Pharmacopoeia" 2005 edition, an appendix VIB thin-layer chromatography test, absorb 5 μl of each of the above two solutions, and point respectively On the same silica gel G thin-layer plate, develop with 60-90°C petroleum ether: ethyl acetate = 20:1 as the developer, take it out, dry it in the air, and spray with 5% p-dimethylaminobenzaldehyde in 10% s...
Embodiment 4
[0229] Embodiment 4 of the present invention: quality control method may comprise the following content:
[0230] Properties: The drug is brown to tan tablets; fragrant, pungent, slightly sweet;
[0231] Identification: (1) Take 4 tablets of this preparation, grind finely, add 20ml of n-hexane, sonicate for 15 minutes, filter, evaporate the filtrate to dryness at low temperature, add 2ml of n-hexane to the residue to dissolve, and use it as the test solution; Medicinal material 0.5g, add n-hexane 2ml, sonicate for 15 minutes, filter, and the filtrate is used as the reference medicinal material solution; according to the "Chinese Pharmacopoeia" 2005 edition, an appendix VIB thin-layer chromatography test, absorb 5 μl of each of the above two solutions, and point respectively On the same silica gel G thin-layer plate, develop with 60-90°C petroleum ether: ethyl acetate = 20:1 as the developer, take it out, dry it in the air, and spray with 5% p-dimethylaminobenzaldehyde in 10% s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com