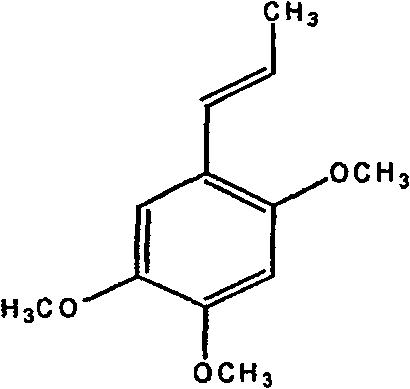
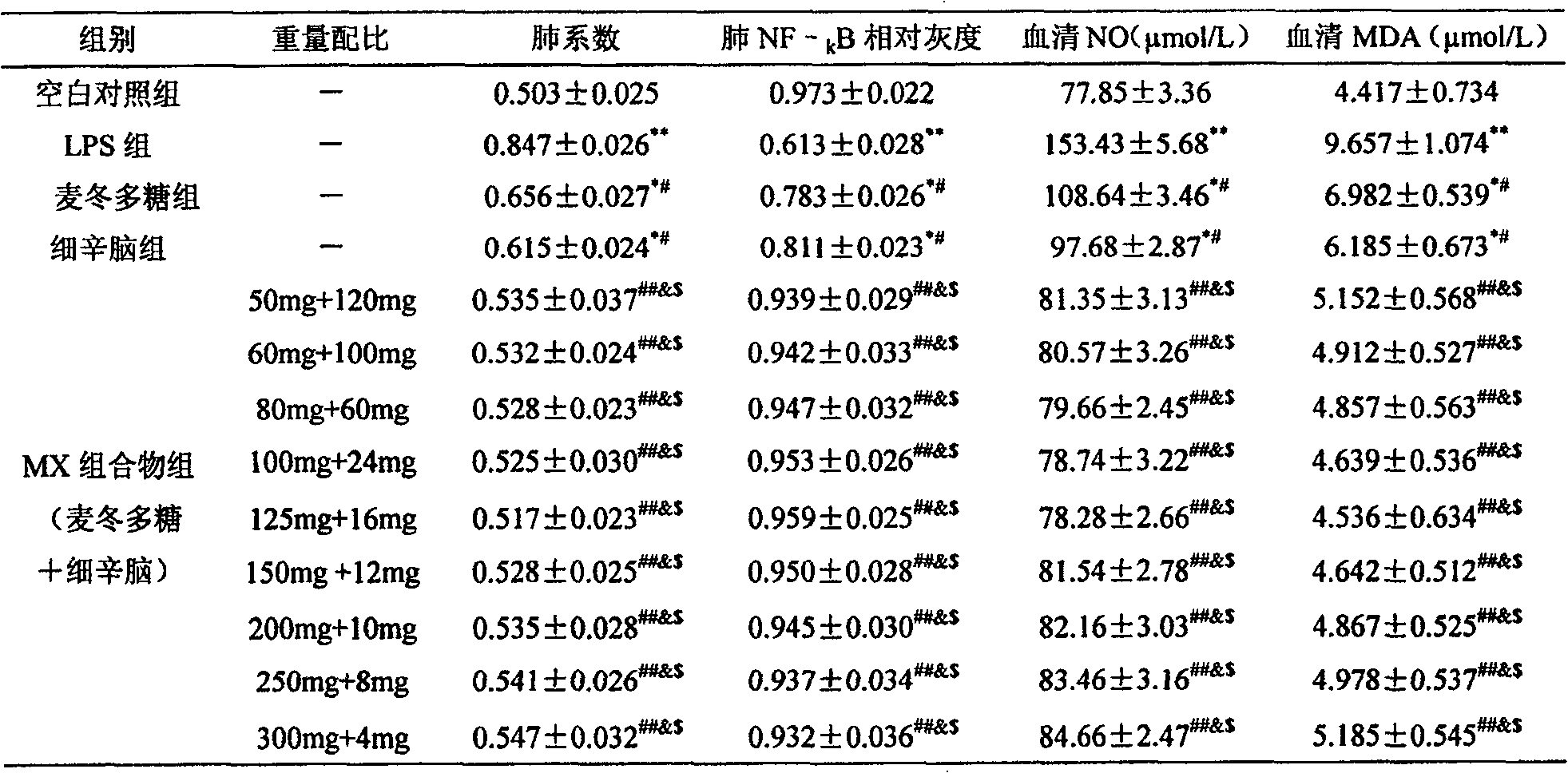
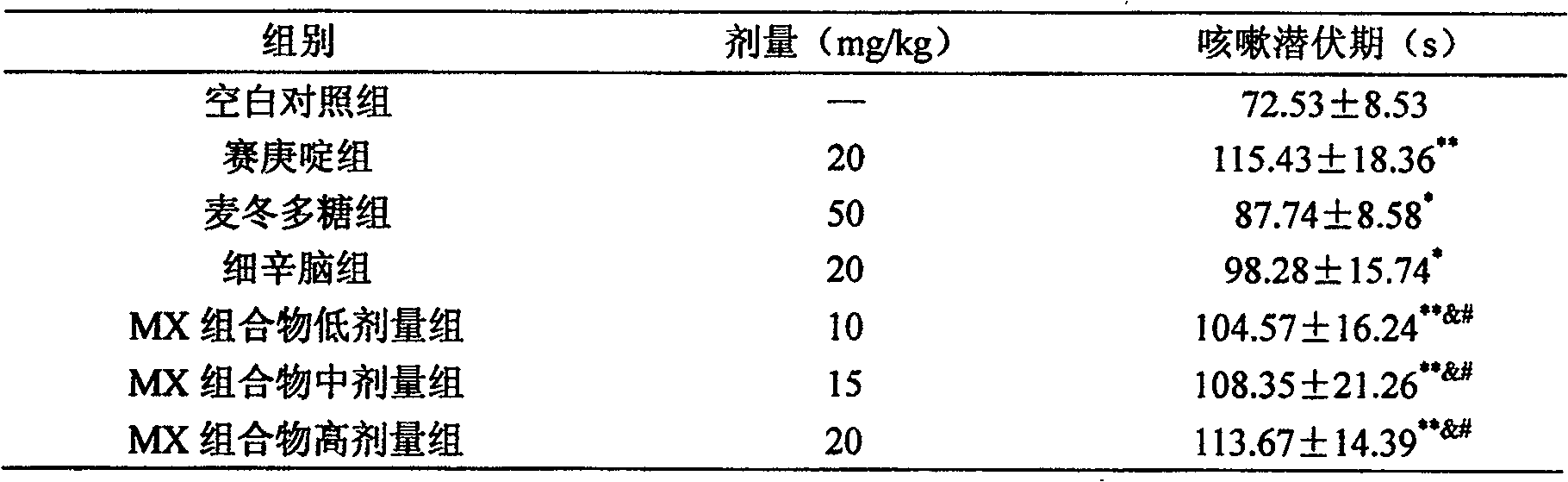
Medicine combination of tuber of dwarf lilyturf and asarone
A composition and technology of Asarum, applied in the field of medicine, achieves the effects of wide application prospect, simple preparation process and guaranteed safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] The preparation of embodiment 1 Ophiopogon japonicus polysaccharide
[0131] Take the dried Ophiopogon japonicus root, extract with ethanol reflux three times, discard the ethanol extract, evaporate the solvent from the dregs, add water to decoct three times, add 6 times the amount of water each time, and the extraction time is 2 hours, 1 hour, and 1 hour respectively. Combine the water extracts, filter, add 0.5% activated carbon for decolorization twice, filter with suction, concentrate the filtrate under reduced pressure to 1 / 5 of the original volume, put it in the refrigerator for more than 24 hours, centrifuge, filter, add ethanol to the filtrate until the alcohol content is 80%, put it in the refrigerator to stand overnight, filter, wash the filter residue several times with absolute ethanol, evaporate the ethanol, add water to heat to dissolve, stand overnight, and centrifuge to remove the precipitate. Adjust the pH of the supernatant to 2.5-5, add 1% pepsin or ...
Embodiment 2
[0144] The preparation of embodiment 2MX composition powder injection
[0145] 1. Prescription:
[0146] Prescription 1:
[0147] Ophiopogon japonicus polysaccharide 125g (equivalent to Ophiopogon japonicus 10kg)
[0148] Asarum 16g
[0149] Polysorbate 80 45g
[0150] Mannitol 500g
[0151] Sorbitol 50g
[0152] Add sterile water for injection to 5000ml
[0153]
[0154] A total of 1000 sticks were prepared
[0155] Prescription 2:
[0156] Ophiopogon japonicus polysaccharide 75g (equivalent to Ophiopogon japonicus 6kg)
[0157] Asarum 90g
[0158] Polysorbate 80 50g
[0159] Mannitol 500g
[0160] Sorbitol 50g
[0161] Add sterile water for injection to 5000ml
[0162]
[0163] A total of 1000 sticks were prepared
[0164] Prescription 3:
[0165] Ophiopogon japonicus polysaccharide 200g (equivalent to Ophiopogon japonicus 16kg)
[0166] Asarum 10g
[0167] Polysorbate 80 100g
[0168] Manni...
Embodiment 3
[0184] The preparation of embodiment 3 composition aqueous injections
[0185] 1. Prescription:
[0186] Prescription 1:
[0187] Ophiopogon japonicus polysaccharide 125g (equivalent to Ophiopogon japonicus 10kg)
[0188] Asarum 16g
[0189] Ethanol 200ml
[0190] Add water for injection to 5000ml
[0191]
[0192] A total of 1000 sticks were prepared
[0193] Prescription 2:
[0194] Ophiopogon japonicus polysaccharide 100g (equivalent to Ophiopogon japonicus 8kg)
[0195] Asarum 24g
[0196] Ethanol 200ml
[0197] Add water for injection to 5000ml
[0198]
[0199] A total of 1000 sticks were prepared
[0200] Prescription 3:
[0201] Ophiopogon japonicus polysaccharide 150g (equivalent to Ophiopogon japonicus 12kg)
[0202] Asarum 12g
[0203] Ethanol 200ml
[0204] Add water for injection to 5000ml
[0205]
[0206] A total of 1000 sticks were prepared
[0207] 2. Speci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com