Method for cloning segment PEXcDNA at 2C end of ground substance metal protease of human from placenta tissue
A placental tissue and matrix metal technology, which is applied in the fields of botanical equipment and methods, biochemical equipment and methods, genetic engineering, etc., to achieve the effect of simple method and good application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
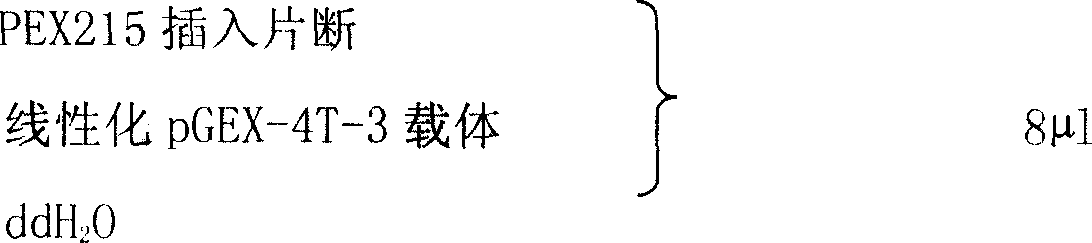
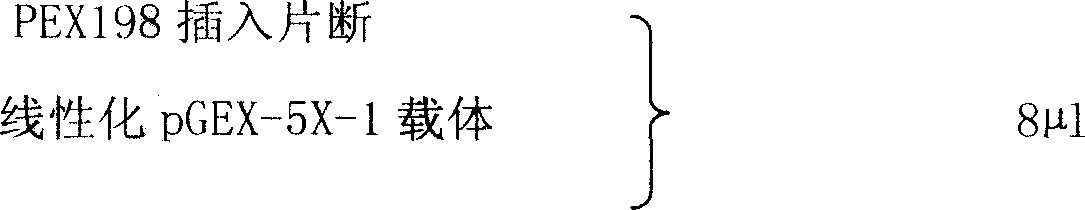
[0017] 1. Extract total RNA from placenta tissue with Qiagen Rneasy Mini Kit.
[0018] 1) Take fresh placental tissue, wash it with normal saline, blot it dry with filter paper, cut it into pieces, put it into a clean pre-cooled mortar, add liquid nitrogen, and grind the tissue block into powder.
[0019] 2) Transfer about 30 mg of tissue into a RNase-free 2 ml Ependorf tube.
[0020] 3) Add 600 μl of RLT buffer solution pre-added with β-mercaptoethanol (β-ME, 10 μl / ml RLT buffer solution), and vortex to lyse the tissue.
[0021] 4) The lysate was centrifuged at room temperature for 3 minutes at the maximum speed of a desktop centrifuge, and the supernatant was transferred to another Ependorf tube.
[0022] 5) Add 600 μl of 70% ethanol, and mix well by pipetting with a pipette tip.
[0023] 6) Put the Rneasy column into a 2ml collection tube, add 700μl of the sample in 5), centrifuge at room temperature at 12000rpm for 15s, and discard the column liquid.
[0024] 7) Put 700...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com