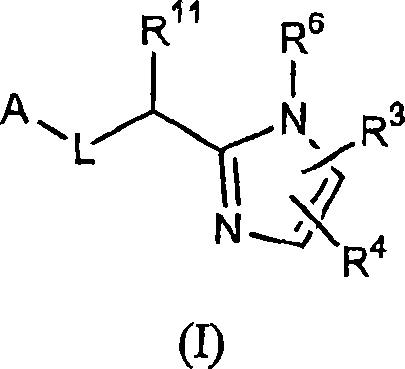
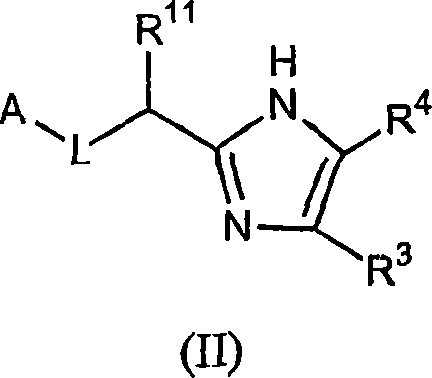
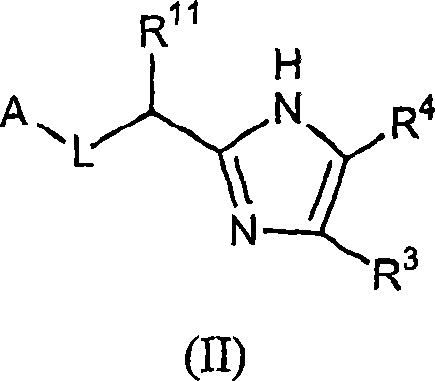
Five-membered heterocycles useful as serine protease inhibitors.
A compound, solvate technology, applied in the field of treatment of thrombotic or inflammatory diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0541] The preparation of prodrugs is well known in the art and is described, for example, in Medicinal Chemistry: Principles and Practice, ed. F.D. King, The Royal Society of Chemistry, Cambridge, UK, 1994, which is hereby incorporated by reference in its entirety.
[0542] Also provided herein are radiolabeled compounds of the invention wherein one or more of said atoms is replaced by a radioactive isotope of that atom (e.g., C is replaced by 13 C or 14 C substitution; isotopes of hydrogen include tritium and deuterium). These compounds have many potential applications, eg, as standards and reagents for determining the ability of potential drugs to bind target proteins or receptors, or to image compounds of the invention bound to biological receptors in vivo or in vitro.
[0543] The compound of the invention is preferably isolated and purified after its preparation to obtain a composition containing 98% or more, preferably 99%, by weight of the compound of the invention ("...
Embodiment 1
[0676] (S)-4-carbamimidoyl-N-(2-phenyl-1-(4-phenyl-1H-imidazol-2-yl)ethyl)benzamide, bis(trifluoroacetic acid) salt
[0677] Step A: (S)-tert-butyl 2-phenyl-1-(4-phenyl-1H-imidazol-2-yl)ethylcarbamate:
[0678] To the EtOH / H of L-N-(Boc)-phenylalanine (750mg, 3.77mmol) 2 Add Cs to O (1:1, 7.4ml) solution 2 CO 3 (650 mg, 1.89 mmol). The reaction was stirred at room temperature for 1 hour. The solvent was removed in vacuo and the resulting salt was suspended in DMF (4.7ml). 2-Bromoacetophenone (1.0 g, 3.77 mmol) was added in one portion and the reaction was stirred at room temperature for 1.5 hours. The reaction was filtered to remove CsBr. The solid was washed with DMF. The combined washings and filtrate were concentrated in vacuo to give a yellow solid (650mg). The crude intermediate was placed in a flask fitted with a Dean-Stark trap and dissolved in xylene (10ml). Add NH to the flask 4 OAc (2.64 g, 30 mmol), and the reaction was heated to reflux for 3 hours. The r...
Embodiment 2
[0685] (S)-4-(aminomethyl)-N-(2-phenyl-1-(4-phenyl-1H-imidazol-2-yl)ethyl)cyclohexanecarboxamide, di-trifluoroacetic acid salt.
[0686] Step A: (S)-(4-((2-Phenyl-1-(4-phenyl-1H-imidazol-2-yl)ethyl)carbamoyl)cyclohexyl)methylcarbamate tert-butyl ester : The product (75mg, 0.15mmol) and N-(Boc)-p-aminomethylcyclohexanecarboxylic acid (tranexamic acid) (75mg, 0.29mmol) in the embodiment 1 step B were dissolved in pyridine (1.7ml) . BOP reagent (152 mg, 0.344 mmol) was added and the reaction was stirred at room temperature for 12 hours. The reaction was diluted with water and extracted with EtOAc. The combined organic layers were washed with brine and washed with Na 2 SO 4 Dry, filter and distill in vacuo. The crude product was used in the next step without further purification. MS 503.2(M+H) + .
[0687] Alternatively, the product from step B of Example 1 was coupled with 1.0 equivalents of N-Boc-p-aminomethylcyclohexanecarboxylic acid, 1.2 equivalents of HOAt, 5.0 equi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com