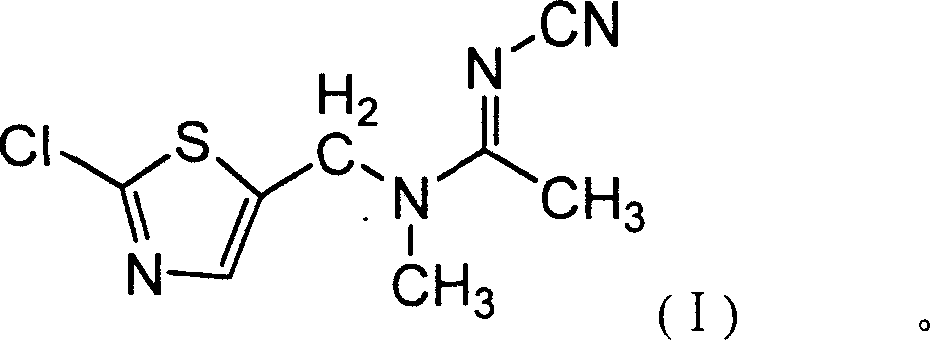
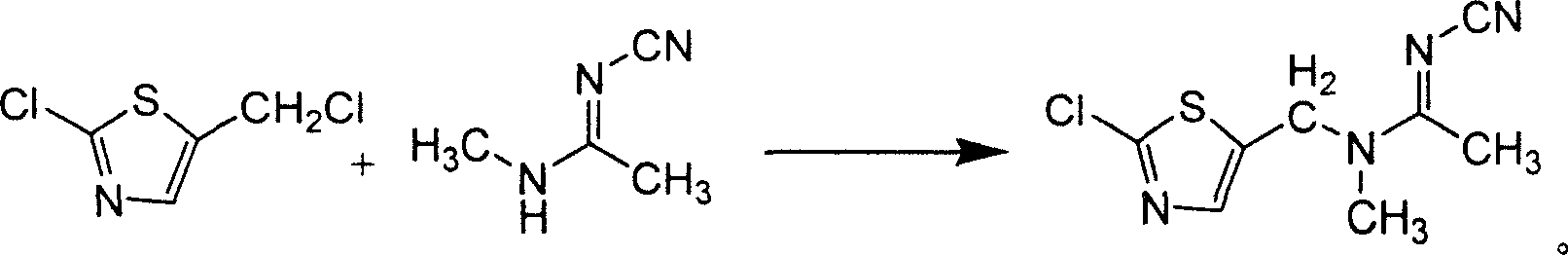Second anabasine insecticide JT-L001 and chemical synthesis method thereof
A technology for pesticides and compounds, applied in the field of organic compounds, can solve the problems of complex process, high technical requirements for preparation, positive temperature effect, etc., and achieves the effects of good environmental compatibility, simple preparation process, and improved quick-acting properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
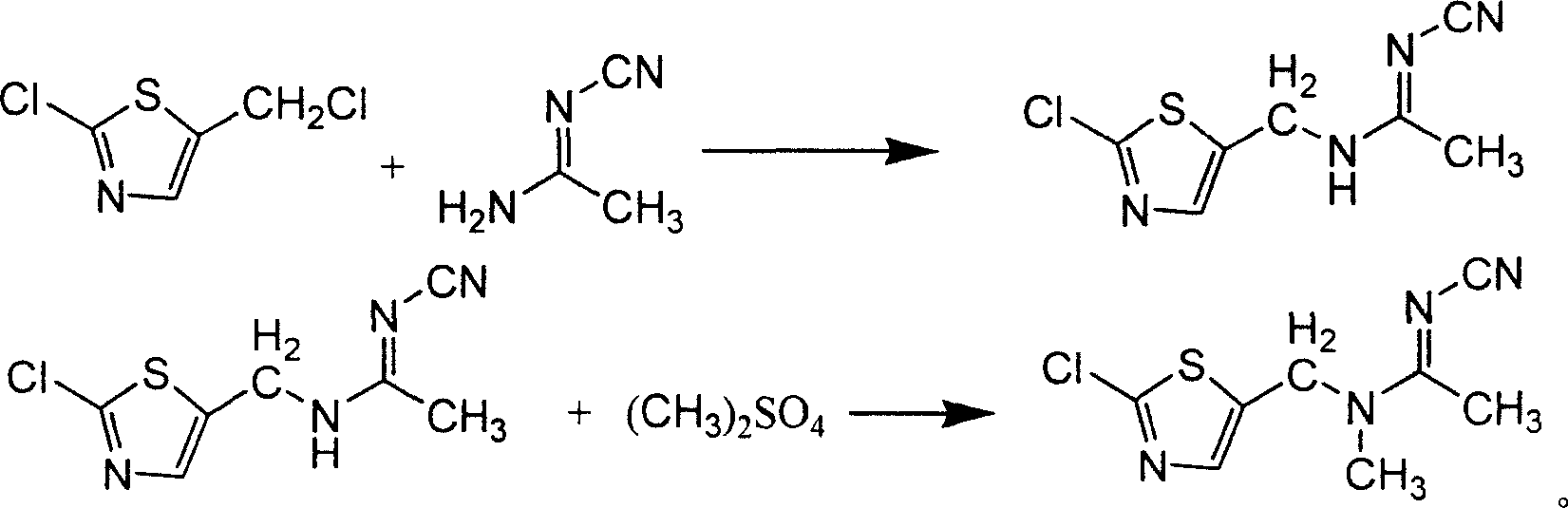
[0040]Add 100ml of absolute ethanol and 0.5g of CsCl to a 500ml reaction flask, stir and add 45.65g (0.55mol) of pure N-cyanoacetamidine, and then slowly add 84g (0.5mol) of pure 2-chloro - Mixed solution of 5-chloromethylthiazole and 50ml of absolute ethanol, keep it dropwise at room temperature for 1 hour, continue to stir for 15 minutes after the dropwise addition, raise the temperature to 70-75°C for half an hour, and discharge non-condensable gas at the same time, Then heat up to reflux, react for 1 hour, slowly cool to <10°C, precipitate crystals, filter and wash the filter cake with ethanol, and dry the crystals to obtain N-cyano-N'-(2-chloro-5-thiazole Base) acetamidine 96.6g, content 89%, yield 80.3%.
[0041] Add the N-cyano-N'-(2-chloro-5-thiazylmethyl)acetamidine (0.401mol) obtained above to the reaction flask, and add 200ml of chloroform, Bu 4 NB r (PTC) 0.03g, stir and cool to 10-15°C, add dropwise 55.5g (0.44mol) of dimethyl sulfate and 50% sodium hydroxide so...
Embodiment 2
[0043] Add 150ml of dried N,N-dimethylformamide into a dry glass reaction bottle, then add 84g (0.5mol) of pure 2-chloro-5-chloromethylthiazole, stir, and then carefully Add 12g (0.5mol) of pure NaH into a reaction flask at 10°C, stir for 15 minutes, then add 48.5g (0.5mol) of pure N-cyano-N'-methylacetamidine, raise the temperature to about 50°C, and stir for 6 Hours, slowly cooled to about 10°C, crystallized, filtered, the filter cake was washed with water, and dried to obtain 173.1g of yellow JT-L00, with a content of 80.2% and a yield of 51.16%.
Embodiment 3
[0045] In a 500ml ground glass reaction bottle, add 84g (0.5mol) of pure product 2-chloro-5-chloromethylthiazole, and dropwise add 59g (1.9mol) of pure product monomethylamine and 95% ethanol under stirring In 150ml pre-refrigerated mixed solution, keep at 0-5°C, stir evenly, raise the temperature to 40°C and react for 4 hours, evaporate ethanol and residual gas under negative pressure, adjust the pH to neutral, pour into a separatory funnel and let it stand for stratification After that, take the oil layer and use anhydrous Na 2 SO 4 After drying, 76.9 g of N-methyl-2-chloro-5-thiazole methylamine was obtained, with a content of 94.1% and a yield of 88.5%.
[0046] Add the mixed solution of 52g (0.46mol) of pure N-cyanoacetate and 100ml of absolute ethanol dropwise into the above reaction flask under stirring, keep the dropping temperature at 40°C, within 2.5h Drop the reaction, then heat up to reflux, react for 3h, cool and crystallize, filter, wash the filter cake with ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com