Novel laccase enzymes and their uses
一种漆酶、发挥作用的技术,应用在应用、酶、氧化还原酶等方向,达到避免环境有害影响的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
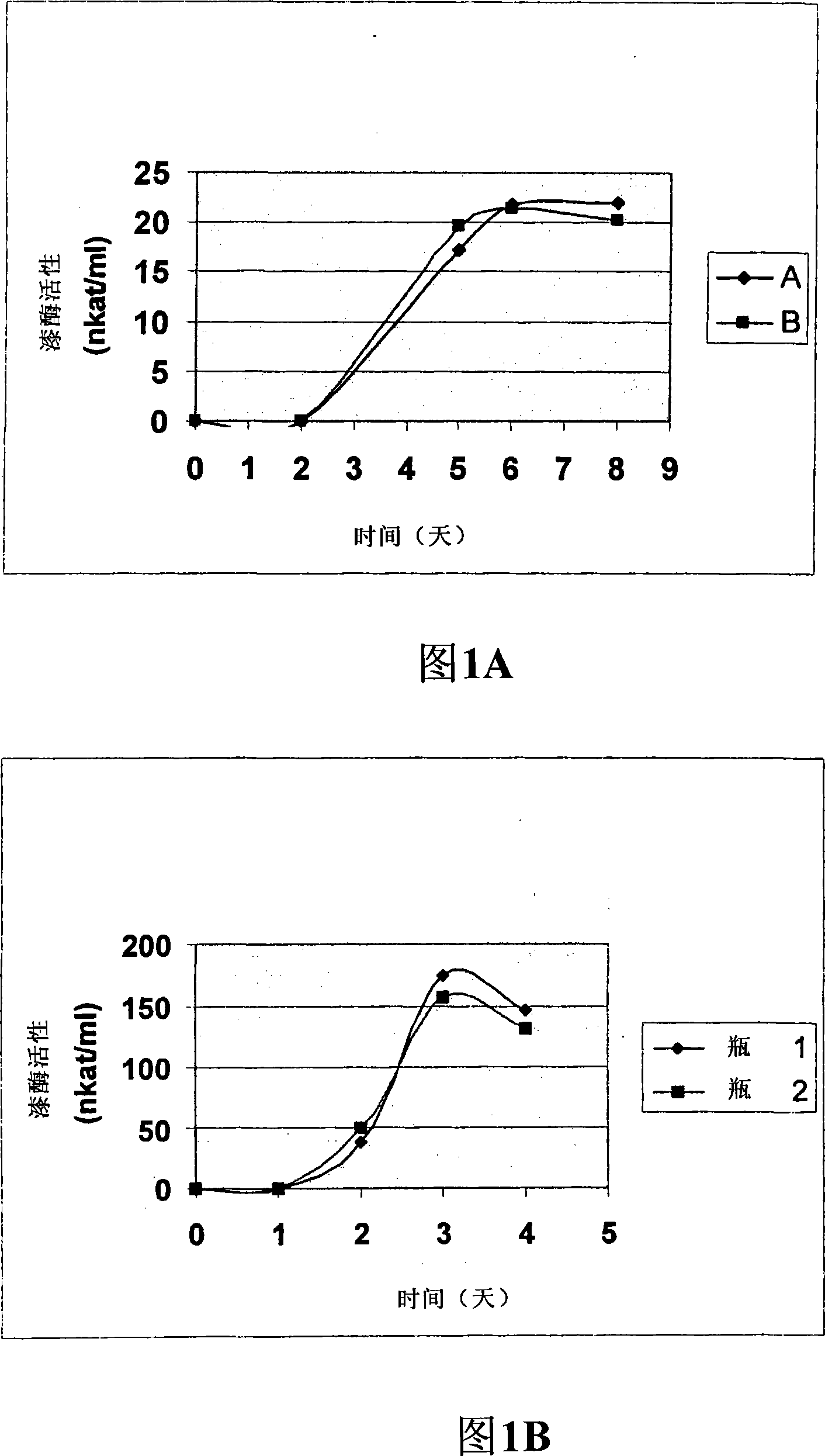
[0137] The purification of preferred laccases of the invention has been exemplified in Example 1. The concentrated culture filtrate of Thielavia was applied to Q Sepharose FF column, the protein was eluted with gradually increasing salt gradient, and the fraction with laccase activity was applied to Sephacryl S100 gel filtration resin. Activity assays were performed after purification followed by SDS-PAGE followed by Coomassie brilliant blue staining. To achieve high purity samples, an additional Resource Q anion exchange step can be included. The culture supernatant of Chaetomium laccase was concentrated by ultrafiltration and buffer changed to binding buffer. The protein was bound to DEAE Sepharose FF and eluted with a gradient of sodium sulfate. The fractions with laccase activity were combined and further purified by hydrophobic interaction chromatography. Finally, the purity of the active fraction was analyzed by SDS-PAGE and Coomassie brilliant blue staining. Naturall...
Embodiment 9
[0204] In Example 9, a test for the stain removal ability of laccases of the invention and Denilite II Base laccase preparations is described. In the tests, grass-stained and tea-stained cloths were artificially soiled, with or without the mediator (methyl syringate). Enzyme doses ranged from 20 to 200 nkat per gram of fabric and tests were carried out at 40, 50 or 60°C and pH 6 for 60 minutes.
[0205] As can be seen in Tables 21 and 22 and Figures 10 to 13, CtLcc1 laccase was effective in removing grass and tea stains at 60°C and TaLcc2 laccase was effective at 50°C in the presence of Mediator. The effect was also seen at 40°C.
[0206] decolorization of dye
[0207] The laccases of the invention can also be used for the decolorization of dyes. Wastewater from printing and dyeing factories, for example, cannot be discharged into natural water bodies without degrading the dyes and / or decolorizing them. Decolorization can be done under the same conditions as used in denim ...
Embodiment 2
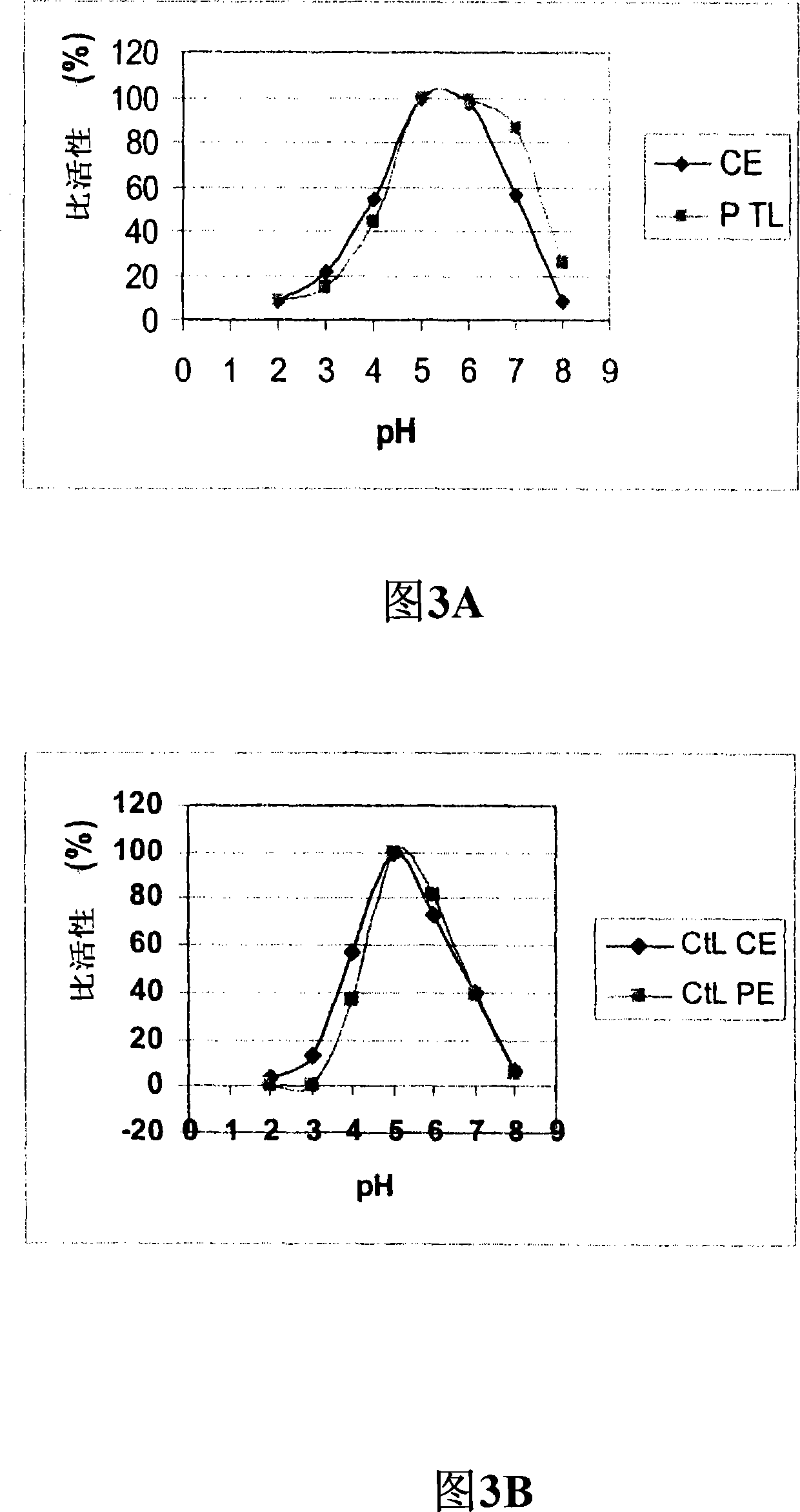
[0238] Example 2: Properties of Purified C. thermophilum Laccase
[0239] molecular weight and isoelectric point
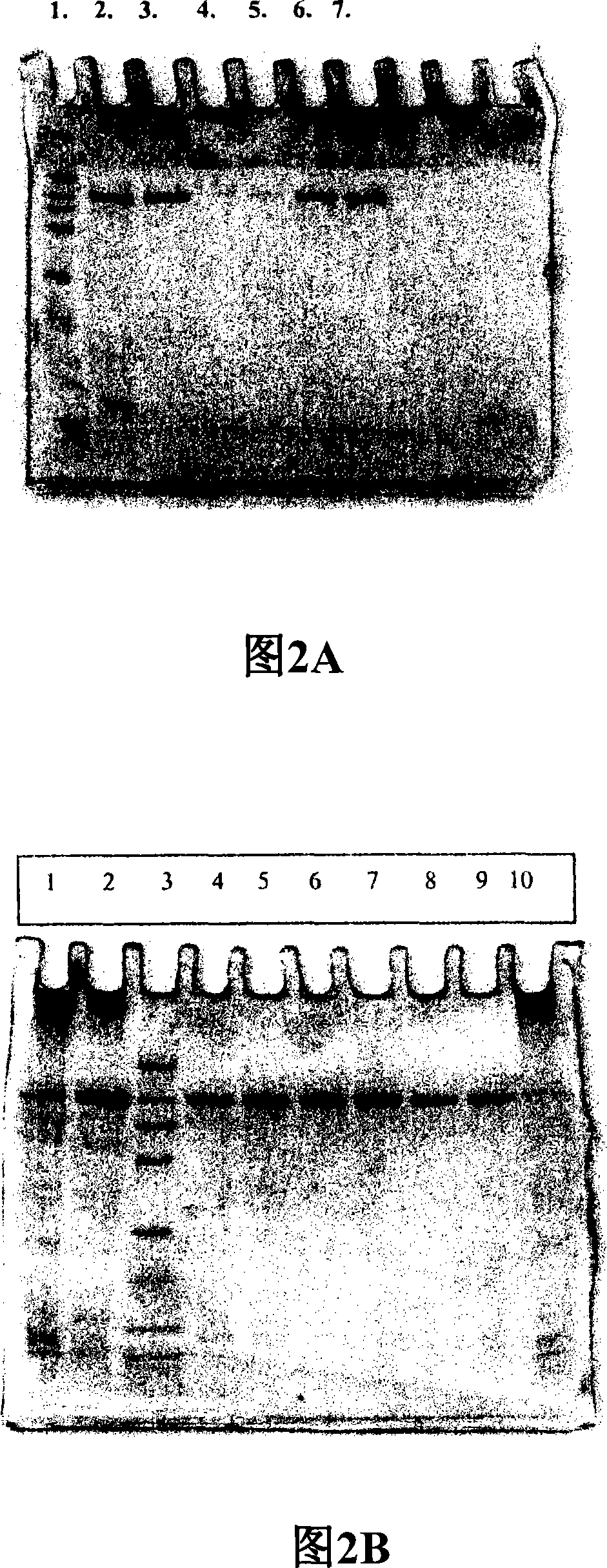
[0240] The molecular weights of T. arenaria and C. thermophilum laccases were determined on SDS-PAGE according to the method of Laemmli (1970). The gel used in the SDS-PAGE analysis was a precast 12% Tris HCl gel (BioRad). Protein bands were visualized by staining with Coomassie Brilliant Blue (R 350; Pharmacia) and compared to molecular weight standards (Prestained Broad Range Molecular Weight Standard #7708 S; New England BioLabs, Beverly, Mass.). Both laccases have a molecular weight of approximately 80 kDa. The isoelectric point of laccase was determined by isoelectric focusing (Pharmalyte IEF, Pharmacia) in the pH range 3-9 using an LKB 2117 Multiphor II electrophoresis system (LKB Pharmacia, Bromma, Sweden) according to the manufacturer's instructions. Bands containing laccase activity were visualized by staining the gel with 2 mM ABTS in 25 mM succinate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com